Respiratory recovery trajectories after severe-to-critical COVID-19: a 1-year prospective multicentre study
- PMID: 36669777
- PMCID: PMC10066566
- DOI: 10.1183/13993003.01532-2022
Respiratory recovery trajectories after severe-to-critical COVID-19: a 1-year prospective multicentre study
Abstract
Background: Survivors of severe-to-critical coronavirus disease 2019 (COVID-19) may have functional impairment, radiological sequelae and persistent symptoms requiring prolonged follow-up. This pragmatic study aimed to describe their clinical follow-up and determine their respiratory recovery trajectories, and the factors that could influence them and their health-related quality of life.
Methods: Adults hospitalised for severe-to-critical COVID-19 were evaluated at 3 months and up to 12 months post-hospital discharge in this prospective, multicentre, cohort study.
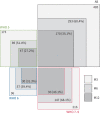
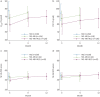
Results: Among 485 enrolled participants, 293 (60%) were reassessed at 6 months and 163 (35%) at 12 months; 89 (51%) and 47 (27%) of the 173 participants initially managed with standard oxygen were reassessed at 6 and 12 months, respectively. At 3 months, 34%, 70% and 56% of the participants had a restrictive lung defect, impaired diffusing capacity of the lung for carbon monoxide (D LCO) and significant radiological sequelae, respectively. During extended follow-up, both D LCO and forced vital capacity percentage predicted increased by means of +4 points at 6 months and +6 points at 12 months. Sex, body mass index, chronic respiratory disease, immunosuppression, pneumonia extent or corticosteroid use during acute COVID-19 and prolonged invasive mechanical ventilation (IMV) were associated with D LCO at 3 months, but not its trajectory thereafter. Among 475 (98%) patients with at least one chest computed tomography scan during follow-up, 196 (41%) had significant sequelae on their last images.
Conclusions: Although pulmonary function and radiological abnormalities improved up to 1 year post-acute COVID-19, high percentages of severe-to-critical disease survivors, including a notable proportion of those managed with standard oxygen, had significant lung sequelae and residual symptoms justifying prolonged follow-up.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: F. Schlemmer reports support for the present manuscript from Fondation du Souffle, consulting fees from Pfizer, lecture honoraria from Gilead, and travel support from Chiesi, GSK, Elivie, Boerhinger Ingelheim, Gilead and Roche, outside the submitted work. P. Le Guen reports support for attending ATS 2022 from Unimed, outside the submitted work. M. Roumila reports grants from Vivisol and AstraZeneca, outside the submitted work. T. Gille reports lecture honoraria from Boehringer Ingelheim and Roche/Genetech, and travel support from Oxyvie, LVL Medical and Vitalaire, outside the submitted work. L. Sésé reports consulting fees from AstraZeneca, lecture honoraria from Boehringer Ingelheim and Roche-Genentech, and travel support from Novartis and Sanofi Aventis, outside the submitted work. Y. Uzunhan reports personal fees from Boehringer Ingelheim, grants and non-financial support from Oxyvie, and personal fees from Roche, outside the submitted work. M. Patout reports grants from Fisher & Paykel, ResMed and Asten Santé, consulting fees from Philips Respironics, ResMed, Asten Santé and GSK, lecture honoraria from Philips Respironics, Asten Santé, ResMed, Air Liquide Medical, SOS Oxygène, Antadir, Chiesi and Jazz Pharmaceutical, travel support from Asten Santé, advisory board participation from ResMed, Philips Respironics and Asten Santé, stock/stock options from Kernel Biomedical, and receipt of equipment/materials from Philips Respironics, ResMed and Fisher & Paykel, outside the submitted work. M. Zysman reports grants from AVAD and INSERM U1045, lecture honoraria from CSL Behring, GSK, Boehringer Ingelheim and AstraZeneca, and travel support Chiesi and AstraZeneca, outside the submitted work. S. Habib reports lecture honoraria from GSK and AstraZeneca, travel support from GSK and Novartis, and advisory board participation with Pfizer, Novartis and Sanofi. C. Jung reports grants from Danone and Menarini, lecture honoraria from Adare and Nestle, and a leadership role and stock/stock options from Biofoodie, outside the submitted work. All other authors have nothing to disclose.
Figures
Comment in
-
Panta rhei (Πάντα ῥεῖ), or everything flows with long COVID.Eur Respir J. 2023 Apr 1;61(4):2202490. doi: 10.1183/13993003.02490-2022. Print 2023 Apr. Eur Respir J. 2023. PMID: 37003610 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
