How I treat HER2-low advanced breast cancer
- PMID: 36669993
- PMCID: PMC9982266
- DOI: 10.1016/j.breast.2023.01.005
How I treat HER2-low advanced breast cancer
Abstract
Introduction: Targeting low levels of human receptor epidermal growth factor 2 (HER2) expression has reshaped the treatment paradigm for half of the patients with advanced breast cancer. HER2-low is currently defined as a HER2 immunohistochemical expression of 1+ or 2+ without amplification by in-situ hybridization. Until recently, HER2-targeted agents were ineffective in treating patients with HER2-low disease.
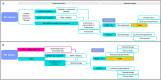
Areas covered: In this narrative review, we summarize the current management of HER2-low breast cancer. We highlight the findings of the DESTINY-Breast 04 phase 3 trial, which confirmed the efficacy of trastuzumab-deruxtecan (T-DXd) for the treatment of patients with advanced, pretreated HER2-low breast cancer. We also discuss how to implement this new treatment option in treatment algorithms of hormone receptor (HR)-positive and triple-negative tumors, as well as how to optimally manage selected toxicities of T-DXd.
Expert opinion: T-DXd is currently the standard of care for patients with advanced, pretreated, HER2-low breast cancer. Based on the design of the DESTINY-Breast04 trial, the current optimal place in treatment algorithms is after the first line of chemotherapy, both in HR-positive and triple-negative breast cancer. Up to 10-15% of the patients receiving T-DXd are expected to develop interstitial lung disease, which in 1-2% of the cases can be fatal. Adequate monitoring and prompt management are required to minimize the impact of ILD and to safely implement T-DXd in clinical practice.
Keywords: Breast cancer; HER2; HER2-Low; Trastuzumab-deruxtecan.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- DeSantis C.E., Ma J., Gaudet M.M., et al. Breast cancer statistics, 2019. CA A Cancer J Clin. 2019;69(6):438–451. - PubMed
-
- Loibl S.J.J., Sonnenblock A., Parlier D., Winer E., et al. VP6-2022: adjuvant pertuzumab and trastuzumab in patients with early HER-2 positive breast cancer in APHINITY: 8.4 years' follow-up. Ann Oncol. 2022:2022. July 15.
-
- Wolff A.C., Hammond M.E.H., Allison K.H., et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36(20):2105–2122. Jul 10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

