Prediction of pathologic complete response to neoadjuvant systemic therapy in triple negative breast cancer using deep learning on multiparametric MRI
- PMID: 36670144
- PMCID: PMC9859781
- DOI: 10.1038/s41598-023-27518-2
Prediction of pathologic complete response to neoadjuvant systemic therapy in triple negative breast cancer using deep learning on multiparametric MRI
Abstract
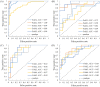
Triple-negative breast cancer (TNBC) is an aggressive subtype of breast cancer. Neoadjuvant systemic therapy (NAST) followed by surgery are currently standard of care for TNBC with 50-60% of patients achieving pathologic complete response (pCR). We investigated ability of deep learning (DL) on dynamic contrast enhanced (DCE) MRI and diffusion weighted imaging acquired early during NAST to predict TNBC patients' pCR status in the breast. During the development phase using the images of 130 TNBC patients, the DL model achieved areas under the receiver operating characteristic curves (AUCs) of 0.97 ± 0.04 and 0.82 ± 0.10 for the training and the validation, respectively. The model achieved an AUC of 0.86 ± 0.03 when evaluated in the independent testing group of 32 patients. In an additional prospective blinded testing group of 48 patients, the model achieved an AUC of 0.83 ± 0.02. These results demonstrated that DL based on multiparametric MRI can potentially differentiate TNBC patients with pCR or non-pCR in the breast early during NAST.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

