Pathomechanisms in central serous chorioretinopathy: A recent update
- PMID: 36670451
- PMCID: PMC9854068
- DOI: 10.1186/s40942-023-00443-2
Pathomechanisms in central serous chorioretinopathy: A recent update
Abstract
Background: Central serous chorioretinopathy (CSCR) is a potentially blinding choroidal disease. Despite decades of research, the pathological mechanisms of CSCR are still poorly understood. In recent years, there has been a strong emphasis on choroidal dysfunction as a primary cause of CSCR.
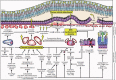
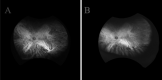
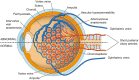
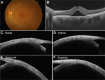
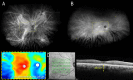
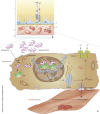
Main body: The concept of the pachychoroid disease spectrum and pachychoroid-driven processes are central to current theories regarding the pathophysiological underpinnings of CSCR. Choroidal hyperpermeability and subsequent leakage of fluid seen in CSCR may be due to several causes. Among them are venous congestion, inflammation, mineralocorticoid receptor activation, systemic factors including hemodynamic changes, obstructive sleep apnea, phosphodiesterase inhibitor use, pregnancy, and genetic predispositions. Congestion of vortex veins that drain blood from the choroid may contribute to the dilation of Haller vessels and cause fluid leakage. Vortex veins exit the eye through the sclera; thus, increased scleral thickness has been proposed to be a factor in venous congestion. Asymmetric vortex vein drainage may similarly result in congestion of the local venous system. Vortex vein anastomoses may overload the venous system and form secondary to venous congestion. Recent studies suggest inflammation and mineralocorticoid activation may factor into the development of CSCR, though more research in these areas is called for. Systemic conditions and genetics may predispose individuals to develop CSCR.
Conclusions: By striving to understand the molecular and physiological mechanisms of this disease, we can better diagnose and treat CSCR to improve outcomes for patients.
Keywords: Central serous chorioretinopathy; Choroid; Pachychoroid disease; Retina.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Chhablani J, Cohen FB, Group CSCI. Multimodal imaging-based central serous chorioretinopathy classification. Ophthalmol Retina 2020; 4:1043–6. 10.1016/j.oret.2020.07.026. - PubMed
-
- Chhablani J, Behar-Cohen F, Group CSCI. Validation of central serous chorioretinopathy multimodal imaging-based classification system. Graefes Arch Clin Exp Ophthalmol 2022; 260:1161–9. 10.1007/s00417-021-05452-1. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

