Long-term exposure to house dust mites accelerates lung cancer development in mice
- PMID: 36670473
- PMCID: PMC9863279
- DOI: 10.1186/s13046-022-02587-9
Long-term exposure to house dust mites accelerates lung cancer development in mice
Abstract
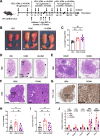
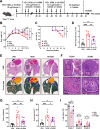
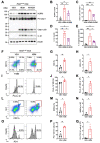
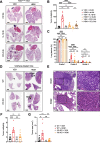
Background: Individuals with certain chronic inflammatory lung diseases have a higher risk of developing lung cancer (LC). However, the underlying mechanisms remain largely unknown. Here, we hypothesized that chronic exposure to house dust mites (HDM), a common indoor aeroallergen associated with the development of asthma, accelerates LC development through the induction of chronic lung inflammation (CLI). METHODS: The effects of HDM and heat-inactivated HDM (HI-HDM) extracts were evaluated in two preclinical mouse models of LC (a chemically-induced model using the carcinogen urethane and a genetically-driven model with oncogenic KrasG12D activation in lung epithelial cells) and on murine macrophages in vitro. Pharmacological blockade or genetic deletion of the Nod-like receptor family pyrin domain-containing protein 3 (NLRP3) inflammasome, caspase-1, interleukin-1β (IL-1β), and C-C motif chemokine ligand 2 (CCL2) or treatment with an inhaled corticosteroid (ICS) was used to uncover the pro-tumorigenic effect of HDM. RESULTS: Chronic intranasal (i.n) instillation of HDM accelerated LC development in the two mouse models. Mechanistically, HDM caused a particular subtype of CLI, in which the NLRP3/IL-1β signaling pathway is chronically activated in macrophages, and made the lung microenvironment conducive to tumor development. The tumor-promoting effect of HDM was significantly decreased by heat treatment of the HDM extract and was inhibited by NLRP3, IL-1β, and CCL2 neutralization, or ICS treatment.
Conclusions: Collectively, these data indicate that long-term exposure to HDM can accelerate lung tumorigenesis in susceptible hosts (e.g., mice and potentially humans exposed to lung carcinogens or genetically predisposed to develop LC).
Keywords: CCL2; Chronic inflammation; House dust mites; IL-1β; Kras; Lung cancer; Macrophages; NLRP3; Tumor microenvironment; Urethane.
© 2023. The Author(s).
Conflict of interest statement
HMH has received speaking fees from Novartis, consulting fees from Novartis, SOBI, Regeneron, and IFM, and research funds from Glaxo Wellcome, Vertex, Burroughs Wellcome, and Jecure. ML, ZA, HS, and DS are employees, shareholders, and/or option holders at Cell IDx. The other authors declare that they have no competing interests.
Figures


References
-
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–454. - PubMed
-
- Gorlova OY, Zhang Y, Schabath MB, Lei L, Zhang Q, Amos CI, et al. Never smokers and lung cancer risk: a case-control study of epidemiological factors. Int J Cancer. 2006;118(7):1798–1804. - PubMed
-
- Malhotra J, Malvezzi M, Negri E, la Vecchia C, Boffetta P. Risk factors for lung cancer worldwide. Eur Respir J. 2016;48(3):889–902. - PubMed
-
- Turner MC, Chen Y, Krewski D, Calle EE, Thun MJ. Chronic obstructive pulmonary disease is associated with lung cancer mortality in a prospective study of never smokers. Am J Respir Crit Care Med. 2007;176(3):285–290. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

