Higher Reduced State of Fe/S-Signals, with the Suppressed Oxidation of P700, Causes PSI Inactivation in Arabidopsis thaliana
- PMID: 36670882
- PMCID: PMC9854443
- DOI: 10.3390/antiox12010021
Higher Reduced State of Fe/S-Signals, with the Suppressed Oxidation of P700, Causes PSI Inactivation in Arabidopsis thaliana
Abstract
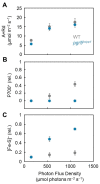
Environmental stress increases the risk of electron accumulation in photosystem I (PSI) of chloroplasts, which can cause oxygen (O2) reduction to superoxide radicals and decreased photosynthetic ability. We used three Arabidopsis thaliana lines: wild-type (WT) and the mutants pgr5hope1 and paa1-7/pox1. These lines have different reduced states of iron/sulfur (Fe/S) signals, including Fx, FA/FB, and ferredoxin, the electron carriers at the acceptor side of PSI. In the dark, short-pulse light was repetitively illuminated to the intact leaves of the plants to provide electrons to the acceptor side of PSI. WT and pgr5hope1 plants showed full reductions of Fe/S during short-pulse light and PSI inactivation. In contrast, paa1-7/pox1 showed less reduction of Fe/S and its PSI was not inactivated. Under continuous actinic-light illumination, pgr5hope1 showed no P700 oxidation with higher Fe/S reduction due to the loss of photosynthesis control and PSI inactivation. These results indicate that the accumulation of electrons at the acceptor side of PSI may trigger the production of superoxide radicals. P700 oxidation, responsible for the robustness of photosynthetic organisms, participates in reactive oxygen species suppression by oxidizing the acceptor side of PSI.
Keywords: Fe/S clusters; P700; ferredoxin; photoinhibition; photosynthetic electron transport; photosystem I.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

