Synthesis and Characterization of a Novel Resveratrol Xylobioside Obtained Using a Mutagenic Variant of a GH10 Endoxylanase
- PMID: 36670947
- PMCID: PMC9855058
- DOI: 10.3390/antiox12010085
Synthesis and Characterization of a Novel Resveratrol Xylobioside Obtained Using a Mutagenic Variant of a GH10 Endoxylanase
Abstract
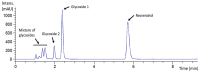
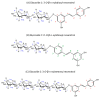
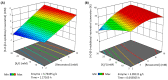
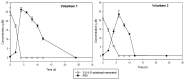
Resveratrol is a natural polyphenol with antioxidant activity and numerous health benefits. However, in vivo application of this compound is still a challenge due to its poor aqueous solubility and rapid metabolism, which leads to an extremely low bioavailability in the target tissues. In this work, rXynSOS-E236G glycosynthase, designed from a GH10 endoxylanase of the fungus Talaromyces amestolkiae, was used to glycosylate resveratrol by using xylobiosyl-fluoride as a sugar donor. The major product from this reaction was identified by NMR as 3-O-ꞵ-d-xylobiosyl resveratrol, together with other glycosides produced in a lower amount as 4'-O-ꞵ-d-xylobiosyl resveratrol and 3-O-ꞵ-d-xylotetraosyl resveratrol. The application of response surface methodology made it possible to optimize the reaction, producing 35% of 3-O-ꞵ-d-xylobiosyl resveratrol. Since other minor glycosides are obtained in addition to this compound, the transformation of the phenolic substrate amounted to 70%. Xylobiosylation decreased the antioxidant capacity of resveratrol by 2.21-fold, but, in return, produced a staggering 4,866-fold improvement in solubility, facilitating the delivery of large amounts of the molecule and its transit to the colon. A preliminary study has also shown that the colonic microbiota is capable of releasing resveratrol from 3-O-ꞵ-d-xylobiosyl resveratrol. These results support the potential of mutagenic variants of glycosyl hydrolases to synthesize highly soluble resveratrol glycosides, which could, in turn, improve the bioavailability and bioactive properties of this polyphenol.
Keywords: antioxidants; colonic fermentation; fungal enzyme; glycoconjugates; glycoside hydrolases; glycosynthase; polyphenols; solubility; xylobiose.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Gambini J., Inglés M., Olaso G., Lopez-Grueso R., Bonet-Costa V., Gimeno-Mallench L., Mas-Bargues C., Abdelaziz K.M., Gomez-Cabrera M.C., Vina J., et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell. Longev. 2015:837042. doi: 10.1155/2015/837042. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

