Use of High-Dose Nebulized Colistimethate in Patients with Colistin-Only Susceptible Acinetobacter baumannii VAP: Clinical, Pharmacokinetic and Microbiome Features
- PMID: 36671325
- PMCID: PMC9855104
- DOI: 10.3390/antibiotics12010125
Use of High-Dose Nebulized Colistimethate in Patients with Colistin-Only Susceptible Acinetobacter baumannii VAP: Clinical, Pharmacokinetic and Microbiome Features
Abstract
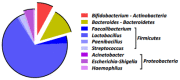
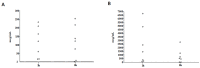
(1) Background: Colistin-only susceptible (COS) Acinetobacter baumannii (AB) ventilator-associated pneumonia (VAP) represents a clinical challenge in the Intensive Care Unit (ICU) due to the negligible lung diffusion of this molecule and the low-grade evidence on efficacy of its nebulization. (2) Methods: We conducted a prospective observational study on 134 ICU patients with COS-AB VAP to describe the 'real life' clinical use of high-dose (5 MIU q8) aerosolized colistin, using a vibrating mesh nebulizer. Lung pharmacokinetics and microbiome features were investigated. (3) Results: Patients were enrolled during the COVID-19 pandemic with the ICU presenting a SAPS II of 42 [32-57]. At VAP diagnosis, the median PaO2/FiO2 was 120 [100-164], 40.3% were in septic shock, and 24.6% had secondary bacteremia. The twenty-eight day mortality was 50.7% with 60.4% and 40.3% rates of clinical cure and microbiological eradication, respectively. We did not observe any drug-related adverse events. Epithelial lining fluid colistin concentrations were far above the CRAB minimal-inhibitory concentration and the duration of nebulized therapy was an independent predictor of microbiological eradication (12 [9.75-14] vs. 7 [4-13] days, OR (95% CI): 1.069 (1.003-1.138), p = 0.039). (4) Conclusions: High-dose and prolonged colistin nebulization, using a vibrating mesh, was a safe adjunctive therapeutic strategy for COS-AB VAP. Its right place and efficacy in this setting warrant investigation in interventional studies.
Keywords: Acinetobacter baumannii; colistin; nebulization; ventilator-associated pneumonia.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Tumbarello M., De Pascale G., Trecarichi E., De Martino S., Bello G., Maviglia R., Spanu T., Antonelli M. Effect of aerosolized colistin as adjunctive treatment on the outcomes of microbiologically documented ventilator-associated pneumonia caused by colistin-only susceptible gram-negative bacteria. Chest. 2013;144:1768–1775. doi: 10.1378/chest.13-1018. - DOI - PubMed
-
- Rocco M., Montini L., Alessandri E., Venditti M., Laderchi A., De Pascale G., Raponi G., Vitale M., Pietropaoli P., Antonelli M. Risk factors for acute kidney injury in critically ill patients receiving high intravenous doses of colistin methanesulfonate and/or other nephrotoxic antibiotics: A retrospective cohort study. Crit. Care. 2013;17:R174. doi: 10.1186/cc12853. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources