New Characterization of Multi-Drug Resistance of Streptococcus suis and Biofilm Formation from Swine in Heilongjiang Province of China
- PMID: 36671333
- PMCID: PMC9854593
- DOI: 10.3390/antibiotics12010132
New Characterization of Multi-Drug Resistance of Streptococcus suis and Biofilm Formation from Swine in Heilongjiang Province of China
Abstract
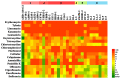
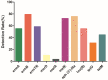
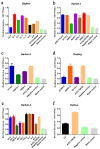
The aim of this study was to investigate the antimicrobial resistance profiles and genotypes of Streptococcus suis in Heilongjiang Province, China. A total of 29 S. suis were isolated from 332 samples collected from 6 pig farms. The results showed that serotypes 2, 4 and 9 were prevalent, and all the clinical isolates were resistant to at least two antibacterial drugs. The most resisted drugs were macrolides, and the least resisted drugs were fluoroquinolones. Resistant genes ermB and aph (3')-IIIa were highly distributed among the isolates, with the detection rates of 79.31% and 75.86%. The formation of biofilm could be observed in all the isolated S. suis, among which D-1, LL-1 and LL-3 strains formed stronger biofilm structure than other strains. The results indicate that S. suis in Heilongjiang Province presents a multi-drug resistance to commonly used antimicrobial drugs, which was caused by the same target gene, the dissemination of drug resistance genes, and bacterial biofilm.
Keywords: Streptococcus suis; biofilm; drug resistance gene; multi-drug resistance; serotype.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Okura M., Takamatsu D., Maruyama F., Nozawa T., Nakagawa I., Osaki M., Sekizaki T., Gottschalk M., Kumagai Y., Hamada S. Genetic analysis of capsular polysaccharide synthesis gene clusters from all serotypes of Streptococcus suis: Potential mechanisms for generation of capsular variation. Appl. Environ. Microbiol. 2013;79:2796–2806. doi: 10.1128/AEM.03742-12. - DOI - PMC - PubMed
-
- Gurung M., Tamang M.D., Moon D.C., Kim S.R., Jeong J.H., Jang G.C., Jung S.C., Park Y.H., Lim S.K. Molecular Basis of Resistance to Selected Antimicrobial Agents in the Emerging Zoonotic Pathogen Streptococcus suis. J. Clin. Microbiol. 2015;53:2332–2336. doi: 10.1128/JCM.00123-15. - DOI - PMC - PubMed
-
- Xu Z., Chen B., Zhang Q., Liu L., Zhang A., Yang Y., Huang K., Yan S., Yu J., Sun X., et al. Streptococcus suis 2 Transcriptional Regulator TstS Stimulates Cytokine Production and Bacteremia to Promote Streptococcal Toxic Shock-Like Syndrome. Front. Microbiol. 2018;9:1309. doi: 10.3389/fmicb.2018.01309. - DOI - PMC - PubMed
-
- Yongkiettrakul S., Maneerat K., Arechanajan B., Malila Y., Srimanote P., Gottschalk M., Visessanguan W. Antimicrobial susceptibility of Streptococcus suis isolated from diseased pigs, asymptomatic pigs, and human patients in Thailand. BMC Vet. Res. 2019;15:5. doi: 10.1186/s12917-018-1732-5. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

