Fluoroquinolones Are Useful as Directed Treatment for Complicated UTI in a Setting with a High Prevalence of Quinolone-Resistant Microorganisms
- PMID: 36671384
- PMCID: PMC9854898
- DOI: 10.3390/antibiotics12010183
Fluoroquinolones Are Useful as Directed Treatment for Complicated UTI in a Setting with a High Prevalence of Quinolone-Resistant Microorganisms
Abstract
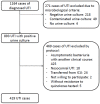
Fluoroquinolones (FQs) have been widely used for treating urinary tract infections (UTIs); however, the increasing emergence of resistant strains has compromised their use. We aimed to know the usefulness of FQs for the treatment of community-acquired UTI in a setting with a high prevalence of fluoroquinolone-resistant microorganisms. A prospective observational study of patients diagnosed with community-acquired UTI was conducted, in which their outcomes according to whether they had FQs or not in their empirical and directed treatments were compared. A multivariate analysis was performed to identify risk factors for UTIs due to ciprofloxacin-resistant microorganisms. A total of 419 patients were included; 162 (38.7%) patients were treated with FQs, as empirical treatment in 27 (6.4%), and as directed treatment in 135 (32.2%). In-hospital mortality (2.2% vs. 6.6%, p 0.044) and 30-day mortality (4.4 vs. 11%, p 0.028) were both lower in the group of patients directly treated with FQ, while there were no differences when FQs were used as empirical treatment. A total of 37.2% of the cases were resistant to ciprofloxacin, which was associated with healthcare-associated UTI (OR 2.7, 95% CI 2-3.7) and prior exposure to FQs (OR 2.7, 95 % CI 1.9-3.7). In conclusion, our findings show that in a setting with a high prevalence of community-acquired UTI caused by quinolone-resistant microorganisms, FQs as directed treatment for community-acquired UTI were associated with better outcomes than other antibiotics, but their use as empirical treatment is not indicated, even in those cases without risk factors for quinolones resistance.
Keywords: community-acquired UTI; fluoroquinolones; outcomes; resistance; risk factors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Gupta K., Hooton T.M., Naber K.G., Wullt B., Colgan R., Miller L.G., Moran G.J., Nicolle L.E., Raz R., Schaeffer A.J., et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011;52:103–120. doi: 10.1093/cid/ciq257. - DOI - PubMed
-
- Sandberg T., Skoog G., Hermansson A.B., Kahlmeter G., Kuylenstierna N., Lannergard A., Otto G., Settergren B., Ekman G.S. Ciprofloxacin for 7 days versus 14 days in women with acute pyelonephritis: A randomised, open-label and double-blind, placebo-controlled, non-inferiority trial. Lancet. 2012;380:484–490. doi: 10.1016/S0140-6736(12)60608-4. - DOI - PubMed
-
- van Nieuwkoop C., van der Starre W.E., Stalenhoef J.E., van Aartrijk A.M., van der Reijden T.J.K., Vollaard A.M., Delfos N.M., van’t Wout J.W., Blom J.W., Spelt I.C., et al. Treatment duration of febrile urinary tract infection: A pragmatic randomized, double-blind, placebo-controlled non-inferiority trial in men and women. BMC Med. 2017;15:70. doi: 10.1186/s12916-017-0835-3. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources