NRF2 in the Epidermal Pigmentary System
- PMID: 36671405
- PMCID: PMC9855619
- DOI: 10.3390/biom13010020
NRF2 in the Epidermal Pigmentary System
Abstract
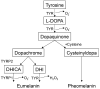
Melanogenesis is a major part of the environmental responses and tissue development of the integumentary system. The balance between reduction and oxidation (redox) governs pigmentary responses, for which coordination among epidermal resident cells is indispensable. Here, we review the current understanding of melanocyte biology with a particular focus on the "master regulator" of oxidative stress responses (i.e., the Kelch-like erythroid cell-derived protein with cap'n'collar homology-associated protein 1-nuclear factor erythroid-2-related factor 2 system) and the autoimmune pigment disorder vitiligo. Our investigation revealed that the former is essential in pigmentogenesis, whereas the latter results from unbalanced redox homeostasis and/or defective intercellular communication in the interfollicular epidermis (IFE). Finally, we propose a model in which keratinocytes provide a "niche" for differentiated melanocytes and may "imprint" IFE pigmentation.
Keywords: NRF2; antioxidant; melanocyte; oxidative stress; reactive oxygen species; redox; vitiligo.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Baicalein protects human vitiligo melanocytes from oxidative stress through activation of NF-E2-related factor2 (Nrf2) signaling pathway.Free Radic Biol Med. 2018 Dec;129:492-503. doi: 10.1016/j.freeradbiomed.2018.10.421. Epub 2018 Oct 18. Free Radic Biol Med. 2018. PMID: 30342186
-
The nuclear factor (erythroid-derived 2)-like 2 (NRF2) antioxidant response promotes melanocyte viability and reduces toxicity of the vitiligo-inducing phenol monobenzone.Exp Dermatol. 2017 Jul;26(7):637-644. doi: 10.1111/exd.13350. Exp Dermatol. 2017. PMID: 28370349 Free PMC article.
-
Nuclear factor erythroid 2-related factor 2 (Nrf2) as a potential therapeutic target for vitiligo.Arch Biochem Biophys. 2020 Dec 15;696:108670. doi: 10.1016/j.abb.2020.108670. Epub 2020 Nov 10. Arch Biochem Biophys. 2020. PMID: 33186606 Review.
-
6-Shogaol Protects Human Melanocytes against Oxidative Stress through Activation of the Nrf2-Antioxidant Response Element Signaling Pathway.Int J Mol Sci. 2020 May 16;21(10):3537. doi: 10.3390/ijms21103537. Int J Mol Sci. 2020. PMID: 32429495 Free PMC article.
-
Melanocytes as instigators and victims of oxidative stress.J Invest Dermatol. 2014 Jun;134(6):1512-1518. doi: 10.1038/jid.2014.65. Epub 2014 Feb 27. J Invest Dermatol. 2014. PMID: 24573173 Free PMC article. Review.
Cited by
-
The alleviating effect of Bai-Ju essence on atopic dermatitis through anti-inflammatory and skin barrier repair mechanisms.Mol Cell Biochem. 2025 May 20. doi: 10.1007/s11010-025-05270-7. Online ahead of print. Mol Cell Biochem. 2025. PMID: 40394445
-
Special Issue "Role of NRF2 in Disease: Novel Molecular Mechanisms and Therapeutic Approaches II".Biomolecules. 2023 May 10;13(5):813. doi: 10.3390/biom13050813. Biomolecules. 2023. PMID: 37238683 Free PMC article.
-
Exploring the Influence of Cold Plasma on Epidermal Melanogenesis In Situ and In Vitro.Int J Mol Sci. 2024 May 10;25(10):5186. doi: 10.3390/ijms25105186. Int J Mol Sci. 2024. PMID: 38791225 Free PMC article.
-
Dimethyl Itaconate Inhibits Melanogenesis in B16F10 Cells.Antioxidants (Basel). 2023 Mar 10;12(3):692. doi: 10.3390/antiox12030692. Antioxidants (Basel). 2023. PMID: 36978940 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

