Raman Spectroscopy on Brain Disorders: Transition from Fundamental Research to Clinical Applications
- PMID: 36671862
- PMCID: PMC9855372
- DOI: 10.3390/bios13010027
Raman Spectroscopy on Brain Disorders: Transition from Fundamental Research to Clinical Applications
Abstract
Brain disorders such as brain tumors and neurodegenerative diseases (NDs) are accompanied by chemical alterations in the tissues. Early diagnosis of these diseases will provide key benefits for patients and opportunities for preventive treatments. To detect these sophisticated diseases, various imaging modalities have been developed such as computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET). However, they provide inadequate molecule-specific information. In comparison, Raman spectroscopy (RS) is an analytical tool that provides rich information about molecular fingerprints. It is also inexpensive and rapid compared to CT, MRI, and PET. While intrinsic RS suffers from low yield, in recent years, through the adoption of Raman enhancement technologies and advanced data analysis approaches, RS has undergone significant advancements in its ability to probe biological tissues, including the brain. This review discusses recent clinical and biomedical applications of RS and related techniques applicable to brain tumors and NDs.
Keywords: Raman spectroscopy; biomarker identification; brain disorders; clinical treatment; statistical analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

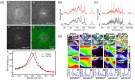



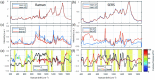
References
-
- Kaufmann T., van der Meer D., Doan N.T., Schwarz E., Lund M.J., Agartz I., Alnæs D., Barch D.M., Baur-Streubel R., Bertolino A., et al. Common brain disorders are associated with heritable patterns of apparent aging of the brain. Nat. Neurosci. 2019;22:1617–1623. doi: 10.1038/s41593-019-0471-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

