Biosensors: Electrochemical Devices-General Concepts and Performance
- PMID: 36671878
- PMCID: PMC9855974
- DOI: 10.3390/bios13010044
Biosensors: Electrochemical Devices-General Concepts and Performance
Abstract
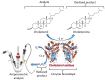
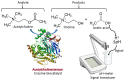
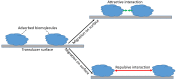
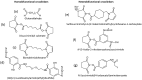
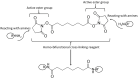
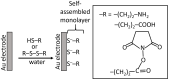
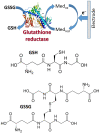
This review provides a general overview of different biosensors, mostly concentrating on electrochemical analytical devices, while briefly explaining general approaches to various kinds of biosensors, their construction and performance. A discussion on how all required components of biosensors are brought together to perform analytical work is offered. Different signal-transducing mechanisms are discussed, particularly addressing the immobilization of biomolecular components in the vicinity of a transducer interface and their functional integration with electronic devices. The review is mostly addressing general concepts of the biosensing processes rather than specific modern achievements in the area.
Keywords: electrochemical biosensors; electron-transfer mediators; enzyme immobilization; enzyme-based biosensors; signal transducers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
















References
-
- Engels J.W., Lottspeich F., editors. Bioanalytics: Analytical Methods and Concepts in Biochemistry and Molecular Biology. Wiley-VCH; Weinheim, Germany: 2018.
-
- Ozkan S.A., Uslu B., Mustafa Kemal Sezgintürk M.K., editors. Biosensors–Fundamentals, Emerging Technologies, and Applications. CRC Press; Boca Raton, FL, USA: 2022.
-
- Ligler F., Taitt C., editors. Optical Biosensors–Today and Tomorrow. Elsevier Science; Amsterdam, The Netherlands: 2008.
-
- Singh P. Electrochemical Biosensors–Applications in Diagnostics, Therapeutics, Environment, and Food Management. Academic Press; Cambridge, MA, USA: 2021.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

