Electrochemical Immunosensor Using Electroactive Carbon Nanohorns for Signal Amplification for the Rapid Detection of Carcinoembryonic Antigen
- PMID: 36671898
- PMCID: PMC9855668
- DOI: 10.3390/bios13010063
Electrochemical Immunosensor Using Electroactive Carbon Nanohorns for Signal Amplification for the Rapid Detection of Carcinoembryonic Antigen
Abstract
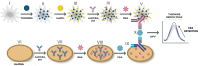
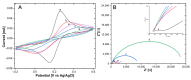
In this work, a novel sandwich-type electrochemical immunosensor was developed for the quantitative detection of the carcinoembryonic antigen, an important tumor marker in clinical tests. The capture antibodies were immobilized on the surface of a gold disk electrode, while detection antibodies were attached to redox-tagged single-walled carbon nanohorns/thionine/AuNPs. Both types of antibody immobilization were carried out through Au-S bonds using the novel photochemical immobilization technique that ensures control over the orientation of the antibodies. The electroactive SWCNH/Thi/AuNPs nanocomposite worked as a signal tag to carry out both the detection of carcinoembryonic antigen and the amplification of the detection signal. The current response was monitored by differential pulse voltammetry. A clear dependence of the thionine redox peak was observed as a function of the carcinoembryonic antigen concentration. A linear detection range from 0.001-200 ng/mL and a low detection limit of 0.1385 pg/mL were obtained for this immunoassay. The results showed that carbon nanohorns represent a promising matrix for signal amplification in sandwich-type electrochemical immune assays working as a conductive and binding matrix with easy and versatile modification routes to antibody and redox tag immobilization, which possesses great potential for clinical diagnostics of CEA and other biomarkers.
Keywords: carbon nanohorns; carcinoembryonic antigen; electrochemical immunosensor; redox-tag.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Hasanzadeh M., Shadjou N., Lin Y., de la Guardia M. Nanomaterials for use in immunosensing of carcinoembryonic antigen (CEA): Recent advances. TrAC—Trends Anal. Chem. 2017;86:185–205. doi: 10.1016/j.trac.2016.11.003. - DOI
-
- Costa R.E., Agustín C.-G. Screen-printed Electrochemical Immunosensors for the Detection of Cancer and Cardiovascular Biomarkers. Electroanalysis. 2016;28:1700–1715. doi: 10.1002/elan.201600126. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

