Evaluation of Endocan as a Treatment for Acute Inflammatory Respiratory Failure
- PMID: 36672192
- PMCID: PMC9857156
- DOI: 10.3390/cells12020257
Evaluation of Endocan as a Treatment for Acute Inflammatory Respiratory Failure
Abstract
Background: Acute respiratory distress syndrome (ARDS) is a life-threatening condition resulting from acute pulmonary inflammation. However, no specific treatment for ARDS has yet been developed. Previous findings suggest that lung injuries related to ARDS could be regulated by endocan (Esm-1). The aim of this study was to evaluate the potential efficiency of endocan in the treatment of ARDS.
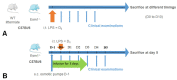
Methods: We first compared the features of acute pulmonary inflammation and the severity of hypoxemia in a tracheal LPS-induced acute lung injury (ALI) model performed in knockout (Esm1-/-) and wild type (WT) littermate C57Bl/6 mice. Next, we assessed the effects of a continuous infusion of glycosylated murine endocan in our ALI model in Esm1-/- mice.
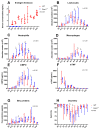
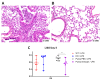
Results: In our ALI model, we report higher alveolar leukocytes (p < 0.001), neutrophils (p < 0.001), and MPO (p < 0.001), and lower blood oxygenation (p < 0.001) in Esm1-/- mice compared to WT mice. Continuous delivery of glycosylated murine endocan after LPS-induced ALI resulted in decreased alveolar leukocytes (p = 0.012) and neutrophils (p = 0.012), higher blood oxygenation levels (p < 0.001), and reduced histological lung injury (p = 0.04), compared to mice treated with PBS.
Conclusions: Endocan appears to be an effective treatment in an ARDS-like model in C57Bl/6 mice.
Keywords: Esm-1; acute lung injury; acute respiratory distress syndrome; endocan; respiratory failure.
Conflict of interest statement
L.P. is a former employee of Biothelis. P.L. is the founder of Biothelis. N.D.F.C is a current employee of Biothelis. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Bellani G., Laffey J.G., Pham T., Fan E., Brochard L., Esteban A., Gattinoni L., van Haren F., Larsson A., McAuley D.F., et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

