Etiological Roles of p75NTR in a Mouse Model of Wet Age-Related Macular Degeneration
- PMID: 36672232
- PMCID: PMC9856885
- DOI: 10.3390/cells12020297
Etiological Roles of p75NTR in a Mouse Model of Wet Age-Related Macular Degeneration
Abstract
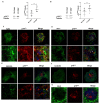
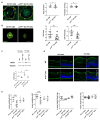
Choroidal neovascularization (CNV) is a pathological angiogenesis of the choroidal plexus of the retina and is a key feature in the wet form of age-related macular degeneration. Mononuclear phagocytic cells (MPCs) are known to accumulate in the subretinal space, generating a chronic inflammatory state that promotes the growth of the choroidal neovasculature. However, how the MPCs are recruited and activated to promote CNV pathology is not fully understood. Using genetic and pharmacological tools in a mouse model of laser-induced CNV, we demonstrate a role for the p75 neurotrophin receptor (p75NTR) in the recruitment of MPCs, in glial activation, and in vascular alterations. After laser injury, expression of p75NTR is increased in activated Muller glial cells near the CNV area in the retina and the retinal pigmented epithelium (RPE)-choroid. In p75NTR knockout mice (p75NTR KO) with CNV, there is significantly reduced recruitment of MPCs, reduced glial activation, reduced CNV area, and the retinal function is preserved, as compared to wild type mice with CNV. Notably, a single intravitreal injection of a pharmacological p75NTR antagonist in wild type mice with CNV phenocopied the results of the p75NTR KO mice. Our results demonstrate that p75NTR is etiological in the development of CNV.
Keywords: choroidal neovascularization; mononuclear phagocytic cells; neurodegeneration; p75NTR; wet age-related macular degeneration.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Effects of p75 neurotrophin receptor on regulating hypoxia-induced angiogenic factors in retinal pigment epithelial cells.Mol Cell Biochem. 2015 Jan;398(1-2):123-34. doi: 10.1007/s11010-014-2212-2. Epub 2014 Sep 9. Mol Cell Biochem. 2015. PMID: 25200140
-
Modulation of p75(NTR) prevents diabetes- and proNGF-induced retinal inflammation and blood-retina barrier breakdown in mice and rats.Diabetologia. 2013 Oct;56(10):2329-39. doi: 10.1007/s00125-013-2998-6. Epub 2013 Aug 7. Diabetologia. 2013. PMID: 23918145 Free PMC article.
-
Evolution of oxidative stress, inflammation and neovascularization in the choroid and retina in a subretinal lipid induced age-related macular degeneration model.Exp Eye Res. 2021 Feb;203:108391. doi: 10.1016/j.exer.2020.108391. Epub 2020 Dec 8. Exp Eye Res. 2021. PMID: 33307075
-
Müller Glial Cells in the Macula: Their Activation and Cell-Cell Interactions in Age-Related Macular Degeneration.Invest Ophthalmol Vis Sci. 2024 Feb 1;65(2):42. doi: 10.1167/iovs.65.2.42. Invest Ophthalmol Vis Sci. 2024. PMID: 38416457 Free PMC article. Review.
-
The functional and molecular roles of p75 neurotrophin receptor (p75NTR) in epilepsy.J Cent Nerv Syst Dis. 2024 Apr 22;16:11795735241247810. doi: 10.1177/11795735241247810. eCollection 2024. J Cent Nerv Syst Dis. 2024. PMID: 38655152 Free PMC article. Review.
References
-
- Wong W.L., Su X., Li X., Cheung C.M., Klein R., Cheng C.Y., Wong T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. - DOI - PubMed
-
- Armstrong R.A., Mousavi M. Overview of Risk Factors for Age-Related Macular Degeneration (AMD) J. Stem Cells. 2015;10:171–191. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

