Triphasic 3D In Vitro Model of Bone-Tendon-Muscle Interfaces to Study Their Regeneration
- PMID: 36672248
- PMCID: PMC9856925
- DOI: 10.3390/cells12020313
Triphasic 3D In Vitro Model of Bone-Tendon-Muscle Interfaces to Study Their Regeneration
Abstract
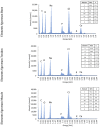
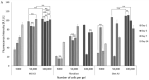
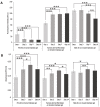
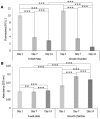
The transition areas between different tissues, known as tissue interfaces, have limited ability to regenerate after damage, which can lead to incomplete healing. Previous studies focussed on single interfaces, most commonly bone-tendon and bone-cartilage interfaces. Herein, we develop a 3D in vitro model to study the regeneration of the bone-tendon-muscle interface. The 3D model was prepared from collagen and agarose, with different concentrations of hydroxyapatite to graduate the tissues from bones to muscles, resulting in a stiffness gradient. This graduated structure was fabricated using indirect 3D printing to provide biologically relevant surface topographies. MG-63, human dermal fibroblasts, and Sket.4U cells were found suitable cell models for bones, tendons, and muscles, respectively. The biphasic and triphasic hydrogels composing the 3D model were shown to be suitable for cell growth. Cells were co-cultured on the 3D model for over 21 days before assessing cell proliferation, metabolic activity, viability, cytotoxicity, tissue-specific markers, and matrix deposition to determine interface formations. The studies were conducted in a newly developed growth chamber that allowed cell communication while the cell culture media was compartmentalised. The 3D model promoted cell viability, tissue-specific marker expression, and new matrix deposition over 21 days, thereby showing promise for the development of new interfaces.
Keywords: 3D cell culture; co-culture; composite hydrogels; indirect 3D printing; regenerative medicine; stiffness gradient; tissue interfaces.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

