Metastatic Prostate Cancer-A Review of Current Treatment Options and Promising New Approaches
- PMID: 36672410
- PMCID: PMC9856730
- DOI: 10.3390/cancers15020461
Metastatic Prostate Cancer-A Review of Current Treatment Options and Promising New Approaches
Abstract
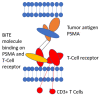
Androgen deprivation therapy (ADT) alone has been the standard of care for many years in men with metastatic prostate cancer. Due to the limited survival under this monotherapy, many new treatment options have been developed in the last few years. Regarding hormone-sensitive prostate cancer, combination therapies of two or three agents of ADT, androgen receptor signaling inhibitors (ARSI) and chemotherapy have been established and led to a significant benefit in overall survival. Additionally, in patients with metastatic castration-resistant prostate cancer, there are many new therapeutic approaches. Chemotherapy alone has been the standard of care in this situation. In the last years, some new therapeutic options have been developed, which led to an improved survival after progression under chemotherapy. These therapies include ARSI, PARP inhibitors and Lu-PSMA radioligand therapy. The use of a bispecific T-cell engager (BiTE) in this setting is a new promising therapeutic approach, which has not been established as standard of care yet. The role of immunotherapy in prostate cancer is still under investigation. Overall, many new treatment options make prostate cancer therapy a challenging and promising field.
Keywords: ADT; PSMA Bite; PSMA radioligand therapy; bispecific T-cell engager; immunotherapy; prostate cancer.
Conflict of interest statement
P.P. has no conflicts of interest; C.D. Travels Fees and Advisory Board from Janssen-Cilag; T.H. has no conflicts of interest; M.W. has no conflicts of interest; B.H. has had advisory roles for ABX, AAA/Novartis, Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Janssen R&D, Lightpoint Medical, Inc., and Pfizer; has received research funding from Astellas, Bristol Myers Squibb, AAA/Novartis, German Research Foundation, Janssen R&D, and Pfizer; and has received compensation for travel from Astellas, AstraZeneca, Bayer and Janssen R&D. KH reports personal fees from Bayer, personal fees and other from Sofie Biosciences, personal fees from SIRTEX, non-financial support from ABX, personal fees from Adacap, personal fees from Curium, personal fees from Endocyte, grants and personal fees from BTG, personal fees from IPSEN, personal fees from Siemens Healthineers, personal fees from GE Healthcare, personal fees from Amgen, personal fees from Novartis, personal fees from ymabs, all outside the submitted work. VG: Receipt of grants/research support: AstraZeneca, Novartis, BMS, MSD, Ipsen, Pfizer; receipt of honoria or consultation fees: AstraZeneca, BMS, Novartis, Apogepha, Ipsen, EISAI, MSD, MerckSerono, Roche, EUSAPharm, Janssen, Nanobiotix, ONO Pharmaceutical, Debiopharm; stock shareholder: AstraZeneca, BMS, SeaGen, MSD, GenMab; travel support: AstraZeneca, BMS, MerckSerono.
Figures
References
-
- Yu E.Y., Klaasen Z. ESM0 2022: Pembrolizumab Plus Olaparib vs. Abiraterone or Enzalutamide for Patients with Previously Treated mCRPC: Randomized Open-Label Phase 3 Keylink 010 Study. [(accessed on 5 January 2023)]. Available online: https://www.urotoday.com/conference-highlights/esmo-2022/esmo-2022-prost....
-
- Slovin S.F., Higano C.S., Hamid O., Tejwani S., Harzstark A., Alumkal J.J., Scher H.I., Chin K., Gagnier P., Mc Henry M.B., et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: Results from an open-label, multicenter phase I/II study. Ann. Oncol. 2013;24:1813–1821. doi: 10.1093/annonc/mdt107. - DOI - PMC - PubMed
-
- Gravis G., Boher J.M., Chen Y.H., Liu G., Fizazi K., Carducci M.A., Oudard S., Joly F., Jarrard D.M., Soulie M., et al. Burden of Metastatic Castrate Naive Prostate Cancer Patients, to Identify Men More Likely to Benefit from Early Docetaxel: Further Analyses of CHAARTED and GETUG-AFU15 Studies. Eur. Urol. 2018;73:847–855. doi: 10.1016/j.eururo.2018.02.001. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous