Libertellenone T, a Novel Compound Isolated from Endolichenic Fungus, Induces G2/M Phase Arrest, Apoptosis, and Autophagy by Activating the ROS/JNK Pathway in Colorectal Cancer Cells
- PMID: 36672439
- PMCID: PMC9857212
- DOI: 10.3390/cancers15020489
Libertellenone T, a Novel Compound Isolated from Endolichenic Fungus, Induces G2/M Phase Arrest, Apoptosis, and Autophagy by Activating the ROS/JNK Pathway in Colorectal Cancer Cells
Abstract
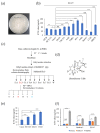
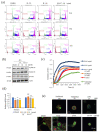
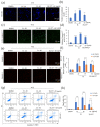
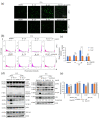
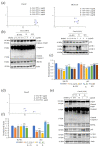
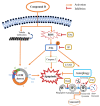
Colorectal cancer (CRC) is the third most deadly type of cancer in the world and continuous investigations are required to discover novel therapeutics for CRC. Induction of apoptosis is one of the promising strategies to inhibit cancers. Here, we have identified a novel compound, Libertellenone T (B), isolated from crude extracts of the endolichenic fungus from Pseudoplectania sp. (EL000327) and investigated the mechanism of action. CRC cells treated by B were subjected to apoptosis detection assays, immunofluorescence imaging, and molecular analyses such as immunoblotting and QRT-PCR. Our findings revealed that B induced CRC cell death via multiple mechanisms including G2/M phase arrest caused by microtubule stabilization and caspase-dependent apoptosis. Further studies revealed that B induced the generation of reactive oxygen species (ROS) attributed to activating the JNK signaling pathway by which apoptosis and autophagy was induced in Caco2 cells. Moreover, B exhibited good synergistic effects when combined with the well-known anticancer drug, 5-FU, and another cytotoxic novel compound D, which was isolated from the same crude extract of EL000327. Overall, Libertellenone T induces G2/M phase arrest, apoptosis, and autophagy via activating the ROS/JNK pathway in CRC. Thus, B may be a potential anticancer therapeutic against CRC that is suitable for clinical applications.
Keywords: CRC; G2/M phase arrest; Libertellenone T; ROS/JNK signaling; apoptosis; autophagy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- ACS Colorectal Cancer Facts and Figures 2020–2022. Am. Cancer Soc. 2020;66:48.
-
- Kellogg J.J., Raja H.A. Endolichenic fungi: A new source of rich bioactive secondary metabolites on the horizon. Phytochem. Rev. 2017;16:271–293. doi: 10.1007/s11101-016-9473-1. - DOI
-
- Yang Y., Bae W.K., Nam S.J., Jeong M.H., Zhou R., Park S.Y., Taş İ., Hwang Y.H., Park M.S., Chung I.J., et al. Acetonic extracts of the endolichenic fungus EL002332 isolated from Endocarpon pusillum exhibits anticancer activity in human gastric cancer cells. Phytomedicine. 2018;40:106–115. doi: 10.1016/j.phymed.2018.01.006. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

