Characteristics and Clinical Outcomes of Patients with Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Receiving Ibrutinib for ≥5 Years in the RESONATE-2 Study
- PMID: 36672456
- PMCID: PMC9857192
- DOI: 10.3390/cancers15020507
Characteristics and Clinical Outcomes of Patients with Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Receiving Ibrutinib for ≥5 Years in the RESONATE-2 Study
Abstract
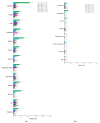
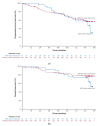
Primary results from the phase 3 RESONATE-2 study demonstrated superior efficacy and tolerability with ibrutinib versus chlorambucil in patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). Here, we describe characteristics and outcomes of patients who received ibrutinib treatment for ≥5 years in RESONATE-2. Patients aged ≥65 years with previously untreated CLL/SLL, without del(17p), were randomly assigned 1:1 to once-daily ibrutinib 420 mg until disease progression/unacceptable toxicity (n = 136) or chlorambucil 0.5−0.8 mg/kg for ≤12 cycles (n = 133). Baseline characteristics in ibrutinib-randomized patients (n = 136) were generally similar between patients on ibrutinib treatment for ≥5 years (n = 79) versus those on treatment for <5 years (n = 57). In patients on ibrutinib treatment for ≥5 years, complete response rates improved over time, reaching 42% by 5 years. Estimated 7-year progression-free survival and overall survival rates were 82% and 94%, respectively. Adverse events (AEs) led to dose reductions in 16/79 patients (20%); these AEs were resolved for 13/16 patients (81%). AEs led to dose holds (≥7 days) in 45/79 patients (57%); these AEs were resolved for 43/45 patients (96%). More than half (58%) of ibrutinib-randomized patients benefitted from ibrutinib treatment for ≥5 years regardless of baseline characteristics. Dose modification resolved AEs for most patients, thereby facilitating continued treatment.
Keywords: chronic lymphocytic leukemia; ibrutinib; long-term outcomes.
Conflict of interest statement
J.A.W.: consulting/advisory role with AbbVie, AstraZeneca, ArQule, BeiGene, Genentech, Janssen, Loxo, Newave, and Pharmacyclics LLC, an AbbVie Company; research funding from AbbVie, Loxo, Karyopharm, MorphoSys, Schrodinger, and Verastem; P.M.B.: consulting/advisory role with AbbVie, AstraZeneca, Bristol Myers Squibb, Celgene, Genentech, Gilead, Janssen, MEI Pharma, Merck, MorphoSys, Pharmacyclics LLC, an AbbVie Company, Seattle Genetics, and TG Therapeutics; research funding from AstraZeneca and TG Therapeutics; T.J.K.: consulting/advisory role with AbbVie, Celgene, Genentech-Roche, Gilead, and Pharmacyclics LLC, an AbbVie Company; research funding from AbbVie, Genentech-Roche, Oncternal Therapeutics, and Pharmacyclics LLC, an AbbVie Company; J.C.B.: honoraria from Janssen; consulting/advisory role for BeiGene, AbbVie, MEI, and AstraZeneca; research funding from Oncternal Therapeutics, Velosbio/Merck, and Pharmacyclics LLC, an AbbVie Company; I.E.A.: no conflicts of interest; P.G.: honoraria from and consulting/advisory role with AbbVie, AstraZeneca, ArQule/Merck Sharp & Dohme, Celgene/Juno/Bristol Myers Squibb, Janssen, Loxo/Lilly, MEI Pharma and Roche; research funding from AbbVie, AstraZeneca, Janssen, and Sunesis; V.G.: employment with Everest Clinical Research; other relationship with AbbVie; E.H.: employment with Pharmacyclics LLC, an AbbVie Company; stock or other ownership with AbbVie; M.J.: employment and stock or other ownership with Pharmacyclics LLC, an AbbVie Company (self) and Gilead Sciences (family member); J.A.B.: honoraria from Gilead, Janssen, Novartis, TG Therapeutics, and Pharmacyclics LLC, an AbbVie Company; consulting/advisory role and speakers bureau for BeiGene, Gilead, Janssen, TG Therapeutics, and Pharmacyclics LLC, an AbbVie Company; research funding from AstraZeneca, BeiGene, and Pharmacyclics LLC; an AbbVie Company; travel/accommodations/expenses from Gilead, Janssen, Novartis, TG Therapeutics, and Pharmacyclics LLC; an AbbVie Company. This study was sponsored by Pharmacyclics LLC, an AbbVie Company. The sponsor was involved in study design, data analysis, data interpretation, writing/review of the manuscript, and the decision to publish the results. The sponsor had no role in data collection.
Figures



References
-
- Barr P.M., Owen C., Robak T., Tedeschi A., Bairey O., Burger J.A., Hillmen P., Coutre S.E., Dearden C., Grosicki S., et al. Up to 8-year follow-up from RESONATE-2: First-line ibrutinib treatment for patients with chronic lymphocytic leukemia. Blood Adv. 2022;6:3440–3450. doi: 10.1182/bloodadvances.2021006434. - DOI - PMC - PubMed
-
- Moreno C., Greil R., Demirkan F., Tedeschi A., Anz B., Larratt L., Simkovic M., Samoilova O., Novak J., Ben-Yehuda D., et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:43–56. doi: 10.1016/S1470-2045(18)30788-5. - DOI - PubMed
-
- Woyach J.A., Ruppert A.S., Heerema N.A., Zhao W., Booth A.M., Ding W., Bartlett N.L., Brander D.M., Barr P.M., Rogers K., et al. Long-term results of alliance A041202 show continued advantage of Ibrutinib-based regimens compared with Bendamustine plus Rituximab (BR) chemoimmunotherapy; Proceedings of the 2021 ASH Meeting and Exhibition; Atlanta, GA, USA. 10–14 December 2021.

