Impact of Liver Metastases and Number of Metastatic Sites on Immune-Checkpoint Inhibitors Efficacy in Patients with Different Solid Tumors: A Retrospective Study
- PMID: 36672591
- PMCID: PMC9855949
- DOI: 10.3390/biomedicines11010083
Impact of Liver Metastases and Number of Metastatic Sites on Immune-Checkpoint Inhibitors Efficacy in Patients with Different Solid Tumors: A Retrospective Study
Abstract
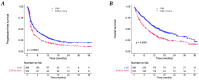
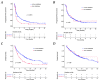
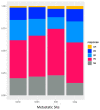
Background: ICIs have dramatically improved patient outcomes in different malignancies. However, the impact of liver metastases (LM) and number of metastatic sites (MS) remains unclear in patients treated with single-agent anti-PD(L)1. Methods: We aimed to assess the prognostic impact of LM and MS number on progression-free survival (PFS) and overall survival (OS) in a large single-arm retrospective multicentric cohort (IMMUCARE) of patients treated with anti-PD(L)-1 for different solid tumors. Results: A total of 759 patients were enrolled from January 2012 to October 2018. The primary tumor types were non-small cell lung cancer (71%), melanoma (19%), or urologic cancer (10%). At the time of ICI initiation, 167 patients (22%) had LM and 370 patients (49%) had more than MS. LM was associated with a shorter median PFS of 1.9 months (95% CI: 1.8−2.5) vs. 4.0 months (95% CI: 3.6−5.4) in patients without LM (p < 0.001). The median OS of patients with LM was of 5.2 months (95% CI: 4.0−7.7) compared with 12.8 months (95% CI: 11.2−15.1) (p < 0.001). Interestingly, LM were not associated with shorter PFS, or OS compared to other MS types (brain, bone, or lung) in patients with only one MS. Patients with multiple MS also had poor clinical outcomes compared to patients with only one MS. The presence of LM and MS number were independent prognostic factors on overall survival. Conclusion: The presence of LM or multiple MS were associated with poorer survival outcomes in patients treated with anti-PD(L)-1.
Keywords: PD1 inhibitors; PDL1 inhibitors; immune checkpoint inhibitors; liver metastases; metastatic sites; prognostic biomarkers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials

