SERPINA3: Stimulator or Inhibitor of Pathological Changes
- PMID: 36672665
- PMCID: PMC9856089
- DOI: 10.3390/biomedicines11010156
SERPINA3: Stimulator or Inhibitor of Pathological Changes
Abstract
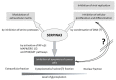
SERPINA3, also called α-1-antichymotrypsin (AACT, ACT), is one of the inhibitors of serine proteases, one of which is cathepsin G. As an acute-phase protein secreted into the plasma by liver cells, it plays an important role in the anti-inflammatory response and antiviral response. Elevated levels of SERPINA3 have been observed in heart failure and neurological diseases such as Alzheimer's disease or Creutzfeldt-Jakob disease. Many studies have shown increased expression levels of the SERPINA3 gene in various types of cancer, such as glioblastoma, colorectal cancer, endometrial cancer, breast cancer, or melanoma. In this case, the SERPINA3 protein is associated with an antiapoptotic function implemented by adjusting the PI3K/AKT or MAPK/ERK 1/2 signal pathways. However, the functions of the SERPINA3 protein are still only partially understood, mainly in the context of cancerogenesis, so it seems necessary to summarize the available information and describe its mechanism of action. In particular, we sought to amass the existing body of research focusing on the description of the underlying mechanisms of various diseases not related to cancer. Our goal was to present an overview of the correct function of SERPINA3 as part of the defense system, which unfortunately easily becomes the "Fifth Column" and begins to support processes of destruction.
Keywords: DNA binding; MAPK/ERK 1/2; PI3K/AKT; SERPINA3; anti-inflammatory; antiapoptotic; α-1-antichymotrypsin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Hwang S.-R., Steineckert B., Kohn A., Palkovits M., Hook V.Y.H. Molecular Studies Define the Primary Structure of A1-Antichymotrypsin (ACT) Protease Inhibitor in Alzheimer’s Disease Brains: Comparison of act in hippocampus and liver*. J. Biol. Chem. 1999;274:1821–1827. doi: 10.1074/jbc.274.3.1821. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

