Delayed Drug Hypersensitivity Reactions: Molecular Recognition, Genetic Susceptibility, and Immune Mediators
- PMID: 36672685
- PMCID: PMC9855900
- DOI: 10.3390/biomedicines11010177
Delayed Drug Hypersensitivity Reactions: Molecular Recognition, Genetic Susceptibility, and Immune Mediators
Abstract
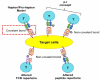
Drug hypersensitivity reactions are classified into immediate and delayed types, according to the onset time. In contrast to the immediate type, delayed drug hypersensitivity mainly involves T lymphocyte recognition of the drug antigens and cell activation. The clinical presentations of such hypersensitivity are various and range from mild reactions (e.g., maculopapular exanthema (MPE) and fixed drug eruption (FDE)), to drug-induced liver injury (DILI) and severe cutaneous adverse reactions (SCARs) (e.g., Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP)). The common culprits of delayed drug hypersensitivity include anti-epileptics, antibiotics, anti-gout agents, anti-viral drugs, etc. Delayed drug hypersensitivity is proposed to be initiated by different models of molecular recognition, composed of drug/metabolite antigen and endogenous peptide, HLA presentation, and T cell receptor (TCR) interaction. Increasing the genetic variants of HLA loci and drug metabolic enzymes has been identified to be responsible for delayed drug hypersensitivity. Furthermore, preferential TCR clonotypes, and the activation of cytotoxic proteins/cytokines/chemokines, are also involved in the pathogenesis of delayed drug hypersensitivity. This review provides a summary of the current understanding of the molecular recognition, genetic susceptibility, and immune mediators of delayed drug hypersensitivity.
Keywords: HLA; Stevens–Johnson syndrome; T lymphocytes; drug hypersensitivity; drug reaction with eosinophilia and systemic symptoms; severe cutaneous adverse reactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- World Health Organization International drug monitoring: The role of national centres. Report of a WHO meeting. World Health Organ Tech. Rep. Ser. 1972;498:1–25. - PubMed
-
- Johansson S.G., Bieber T., Dahl R., Friedmann P.S., Lanier B.Q., Lockey R.F., Motala C., Ortega Martell J.A., Platts-Mills T.A., Ring J., et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J. Allergy Clin. Immunol. 2004;113:832–836. doi: 10.1016/j.jaci.2003.12.591. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

