Timeline of Developmental Defects Generated upon Genetic Inhibition of the Retinoic Acid Receptor Signaling Pathway
- PMID: 36672706
- PMCID: PMC9856201
- DOI: 10.3390/biomedicines11010198
Timeline of Developmental Defects Generated upon Genetic Inhibition of the Retinoic Acid Receptor Signaling Pathway
Abstract
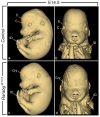
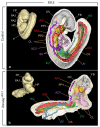
It has been established for almost 30 years that the retinoic acid receptor (RAR) signalling pathway plays essential roles in the morphogenesis of a large variety of organs and systems. Here, we used a temporally controlled genetic ablation procedure to precisely determine the time windows requiring RAR functions. Our results indicate that from E8.5 to E9.5, RAR functions are critical for the axial rotation of the embryo, the appearance of the sinus venosus, the modelling of blood vessels, and the formation of forelimb buds, lung buds, dorsal pancreatic bud, lens, and otocyst. They also reveal that E9.5 to E10.5 spans a critical developmental period during which the RARs are required for trachea formation, lung branching morphogenesis, patterning of great arteries derived from aortic arches, closure of the optic fissure, and growth of inner ear structures and of facial processes. Comparing the phenotypes of mutants lacking the 3 RARs with that of mutants deprived of all-trans retinoic acid (ATRA) synthesising enzymes establishes that cardiac looping is the earliest known morphogenetic event requiring a functional ATRA-activated RAR signalling pathway.
Keywords: HREM; axial rotation; cardiac looping; embryonic turning; eye development; heart development; inner ear development; lung development; mouse.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Mark M., Ghyselinck N.B., Chambon P. Function of retinoid nuclear receptors: Lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu. Rev. Pharmacol. Toxicol. 2006;46:451–480. doi: 10.1146/annurev.pharmtox.46.120604.141156. - DOI - PubMed
-
- Ruzankina Y., Pinzon-Guzman C., Asare A., Ong T., Pontano L., Cotsarelis G., Zediak V.P., Velez M., Bhandoola A., Brown E.J. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1:113–126. doi: 10.1016/j.stem.2007.03.002. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

