Drosophila melanogaster as a Model to Study Fragile X-Associated Disorders
- PMID: 36672829
- PMCID: PMC9859539
- DOI: 10.3390/genes14010087
Drosophila melanogaster as a Model to Study Fragile X-Associated Disorders
Abstract
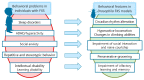
Fragile X syndrome (FXS) is a global neurodevelopmental disorder caused by the expansion of CGG trinucleotide repeats (≥200) in the Fragile X Messenger Ribonucleoprotein 1 (FMR1) gene. FXS is the hallmark of Fragile X-associated disorders (FXD) and the most common monogenic cause of inherited intellectual disability and autism spectrum disorder. There are several animal models used to study FXS. In the FXS model of Drosophila, the only ortholog of FMR1, dfmr1, is mutated so that its protein is missing. This model has several relevant phenotypes, including defects in the circadian output pathway, sleep problems, memory deficits in the conditioned courtship and olfactory conditioning paradigms, deficits in social interaction, and deficits in neuronal development. In addition to FXS, a model of another FXD, Fragile X-associated tremor/ataxia syndrome (FXTAS), has also been established in Drosophila. This review summarizes many years of research on FXD in Drosophila models.
Keywords: Drosophila melanogaster; FMR1 gene; FMRP; FXTAS; Fragile X syndrome.
Conflict of interest statement
Dejan B. Budimirovic has received funding from Zynerba Pharmaceuticals (ZYN2-CL-017, ZYN2-CL-033) as a principal investigator on clinical trials in FXS. He also consulted on clinical trial outcome measures in FXS (Seaside, Ovid). All the above funding has been directed to the Kennedy Krieger Institute/the Johns Hopkins Medical Institutions; Dejan B. Budimirovic receives no personal funds, and the Institute has no relevant financial interest in any of the commercial entities listed. Other authors declare no conflict of interest.
Figures



References
-
- King R.C. Genetics. 2nd ed. Oxford University Press; New York, NY, USA: 1965. pp. 1–462.
-
- Fuentes E., Sivakumar N., Selvik L.-K., Arch M., Cardona P.J., Ioerger T.R., Dragset M.S. Drosophila melanogaster is a powerful host model to study mycobacterial virulence. bioRxiv. 2022 doi: 10.1101/2022.05.12.491628. - DOI
-
- Russell W.M.S., Burch R.L. The Principles of Humane Experimental Technique. Methuen & Co, Ltd.; North Yorkshire, UK: 1959. p. 238.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

