The Yin-Yang Pharmacomicrobiomics on Treatment Response in Inflammatory Arthritides: A Narrative Review
- PMID: 36672830
- PMCID: PMC9859330
- DOI: 10.3390/genes14010089
The Yin-Yang Pharmacomicrobiomics on Treatment Response in Inflammatory Arthritides: A Narrative Review
Abstract
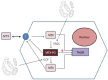
(1) Background: Gut microbiota (GM) is the set of microorganisms inhabiting the gastroenteric tract that seems to have a role in the pathogenesis of rheumatic diseases. Recently, many authors proved that GM may influence pharmacodynamics and pharmacokinetics of several drugs with complex interactions that are studied by the growing field of pharmacomicrobiomics. The aim of this review is to highlight current evidence on pharmacomicrobiomics applied to the main treatments of Rheumatoid Arthritis and Spondyloarthritis in order to maximize therapeutic success, in the framework of Personalized Medicine. (2) Methods: We performed a narrative review concerning pharmacomicrobiomics in inflammatory arthritides. We evaluated the influence of gut microbiota on treatment response of conventional Disease Modifying Anti-Rheumatic drugs (cDMARDs) (Methotrexate and Leflunomide) and biological Disease Modifying Anti-Rheumatic drugs (bDMARDs) (Tumor necrosis factor inhibitors, Interleukin-17 inhibitors, Interleukin 12/23 inhibitors, Abatacept, Janus Kinase inhibitors and Rituximab). (3) Results: We found a great amount of studies concerning Methotrexate and Tumor Necrosis Inhibitors (TNFi). Conversely, fewer data were available about Interleukin-17 inhibitors (IL-17i) and Interleukin 12/23 inhibitors (IL-12/23i), while none was identified for Janus Kinase Inhibitors (JAKi), Tocilizumab, Abatacept and Rituximab. We observed that microbiota and drugs are influenced in a mutual and reciprocal way. Indeed, microbiota seems to influence therapeutic response and efficacy, whereas in the other hand, drugs may restore healthy microbiota. (4) Conclusions: Future improvement in pharmacomicrobiomics could help to detect an effective biomarker able to guide treatment choice and optimize management of inflammatory arthritides.
Keywords: gut microbiota; inflammatory arthritides; personalized medicine; pharmacomicrobiomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

