Degradation of Agro-Industrial Wastewater Model Compound by UV-A-Fenton Process: Batch vs. Continuous Mode
- PMID: 36674030
- PMCID: PMC9858821
- DOI: 10.3390/ijerph20021276
Degradation of Agro-Industrial Wastewater Model Compound by UV-A-Fenton Process: Batch vs. Continuous Mode
Abstract
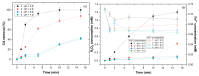
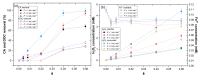
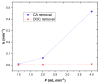
The degradation of a model agro-industrial wastewater phenolic compound (caffeic acid, CA) by a UV-A-Fenton system was investigated in this work. Experiments were carried out in order to compare batch and continuous mode. Initially, batch experiments showed that UV-A-Fenton at pH 3.0 (pH of CA solution) achieved a higher generation of HO•, leading to high CA degradation (>99.5%). The influence of different operational conditions, such as H2O2 and Fe2+ concentrations, were evaluated. The results fit a pseudo first-order (PFO) kinetic model, and a high kinetic rate of CA removal was observed, with a [CA] = 5.5 × 10−4 mol/L, [H2O2] = 2.2 × 10−3 mol/L and [Fe2+] = 1.1 × 10−4 mol/L (kCA = 0.694 min−1), with an electric energy per order (EEO) of 7.23 kWh m−3 order−1. Under the same operational conditions, experiments in continuous mode were performed under different flow rates. The results showed that CA achieved a steady state with higher space-times (θ = 0.04) in comparison to dissolved organic carbon (DOC) removal (θ = 0−0.020). The results showed that by increasing the flow rate (F) from 1 to 4 mL min−1, the CA and DOC removal rate increased significantly (kCA = 0.468 min−1; kDOC = 0.00896 min−1). It is concluded that continuous modes are advantageous systems that can be adapted to wastewater treatment plants for the treatment of real agro-industrial wastewaters.
Keywords: UV-A LEDs; caffeic acid; electric energy per order; environmental impact; photo-Fenton; winery wastewater.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Alayu E., Yirgu Z. Advanced technologies for the treatment of wastewaters from agro-processing industries and cogeneration of by-products: A case of slaughterhouse, dairy and beverage industries. Int. J. Environ. Sci. Technol. 2018;15:1581–1596. doi: 10.1007/s13762-017-1522-9. - DOI
-
- Rajagopal R., Saady N.M.C., Torrijos M., Thanikal J.V., Hung Y.T. Sustainable agro-food industrial wastewater treatment using high rate anaerobic process. Water. 2013;5:292–311. doi: 10.3390/w5010292. - DOI
-
- Amor C., Marchão L., Lucas M.S., Peres J.A. Application of advanced oxidation processes for the treatment of recalcitrant agro-industrial wastewater: A review. Water. 2019;11:205. doi: 10.3390/w11020205. - DOI
-
- Krzemińska D., Neczaj E., Borowski G. Advanced oxidation processes for food industrial wastewater decontamination. J. Ecol. Eng. 2015;16:61–71. doi: 10.12911/22998993/1858. - DOI
-
- Spennati E., Casazza A.A., Converti A. Winery wastewater treatment by microalgae to production purposes. Energies. 2020;13:2490. doi: 10.3390/en13102490. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

