Mendelian Randomisation Confirms the Role of Y-Chromosome Loss in Alzheimer's Disease Aetiopathogenesis in Men
- PMID: 36674414
- PMCID: PMC9863537
- DOI: 10.3390/ijms24020898
Mendelian Randomisation Confirms the Role of Y-Chromosome Loss in Alzheimer's Disease Aetiopathogenesis in Men
Abstract
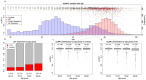
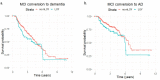
Mosaic loss of chromosome Y (mLOY) is a common ageing-related somatic event and has been previously associated with Alzheimer's disease (AD). However, mLOY estimation from genotype microarray data only reflects the mLOY degree of subjects at the moment of DNA sampling. Therefore, mLOY phenotype associations with AD can be severely age-confounded in the context of genome-wide association studies. Here, we applied Mendelian randomisation to construct an age-independent mLOY polygenic risk score (mloy-PRS) using 114 autosomal variants. The mloy-PRS instrument was associated with an 80% increase in mLOY risk per standard deviation unit (p = 4.22 × 10-20) and was orthogonal with age. We found that a higher genetic risk for mLOY was associated with faster progression to AD in men with mild cognitive impairment (hazard ratio (HR) = 1.23, p = 0.01). Importantly, mloy-PRS had no effect on AD conversion or risk in the female group, suggesting that these associations are caused by the inherent loss of the Y chromosome. Additionally, the blood mLOY phenotype in men was associated with increased cerebrospinal fluid levels of total tau and phosphorylated tau181 in subjects with mild cognitive impairment and dementia. Our results strongly suggest that mLOY is involved in AD pathogenesis.
Keywords: Alzheimer’s disease; CSF biomarkers; EADB; GR@ACE/DEGESCO; GWAS; Mendelian randomization; disease progression; mild cognitive impairment; mosaic loss of chromosome Y; polygenic risk score.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Strittmatter W.J., Saunders A.M., Schmechel D., Pericak-Vance M., Enghild J., Salvesen G.S., Roses A.D. Apolipoprotein E: High-Avidity Binding to β-Amyloid and Increased Frequency of Type 4 Allele in Late-Onset Familial Alzheimer Disease. Proc. Natl. Acad. Sci. USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

