Multi-Omic Factors Associated with Frequency of Upper Respiratory Infections in Developing Infants
- PMID: 36674462
- PMCID: PMC9860840
- DOI: 10.3390/ijms24020934
Multi-Omic Factors Associated with Frequency of Upper Respiratory Infections in Developing Infants
Abstract
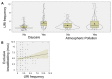
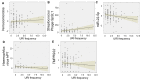
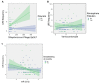
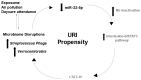
Susceptibility to upper respiratory infections (URIs) may be influenced by host, microbial, and environmental factors. We hypothesized that multi-omic analyses of molecular factors in infant saliva would identify complex host-environment interactions associated with URI frequency. A cohort study involving 146 infants was used to assess URI frequency in the first year of life. Saliva was collected at 6 months for high-throughput multi-omic measurement of cytokines, microRNAs, transcripts, and microbial RNA. Regression analysis identified environmental (daycare attendance, atmospheric pollution, breastfeeding duration), microbial (Verrucomicrobia, Streptococcus phage), and host factors (miR-22-5p) associated with URI frequency (p < 0.05). These results provide pathophysiologic clues about molecular factors that influence URI susceptibility. Validation of these findings in a larger cohort could one day yield novel approaches to detecting and managing URI susceptibility in infants.
Keywords: infants; miRNA; multi-omic; upper respiratory infections; viral infections.
Conflict of interest statement
S.D.H. is a consultant and advisory board member for Quadrant Biosciences and Spectrum Solutions, who played no role in the current study. The other authors have no financial relationships to disclose. The study does not discuss an investigative use of a commercial product.
Figures


References
-
- Shahan B., Barstow C., Mahowald M. Respiratory Conditions: Upper Respiratory Tract Infections. FP Essent. 2019;486:11–18. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

