Experimental and Bioinformatic Insights into the Effects of Epileptogenic Variants on the Function and Trafficking of the GABA Transporter GAT-1
- PMID: 36674476
- PMCID: PMC9862756
- DOI: 10.3390/ijms24020955
Experimental and Bioinformatic Insights into the Effects of Epileptogenic Variants on the Function and Trafficking of the GABA Transporter GAT-1
Abstract
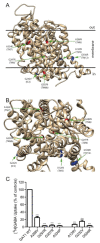
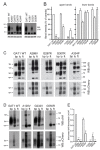
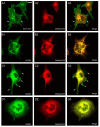
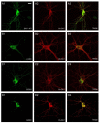
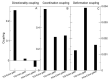
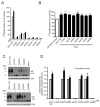
In this article, we identified a novel epileptogenic variant (G307R) of the gene SLC6A1, which encodes the GABA transporter GAT-1. Our main goal was to investigate the pathogenic mechanisms of this variant, located near the neurotransmitter permeation pathway, and compare it with other variants located either in the permeation pathway or close to the lipid bilayer. The mutants G307R and A334P, close to the gates of the transporter, could be glycosylated with variable efficiency and reached the membrane, albeit inactive. Mutants located in the center of the permeation pathway (G297R) or close to the lipid bilayer (A128V, G550R) were retained in the endoplasmic reticulum. Applying an Elastic Network Model, to these and to other previously characterized variants, we found that G307R and A334P significantly perturb the structure and dynamics of the intracellular gate, which can explain their reduced activity, while for A228V and G362R, the reduced translocation to the membrane quantitatively accounts for the reduced activity. The addition of a chemical chaperone (4-phenylbutyric acid, PBA), which improves protein folding, increased the activity of GAT-1WT, as well as most of the assayed variants, including G307R, suggesting that PBA might also assist the conformational changes occurring during the alternative access transport cycle.
Keywords: 4-phenylbutyrate; GABA transporter; epilepsy; intracellular trafficking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Carvill G.L., McMahon J.M., Schneider A., Zemel M., Myers C.T., Saykally J., Nguyen J., Robbiano A., Zara F., Specchio N., et al. Mutations in the GABA Transporter SLC6A1 Cause Epilepsy with Myoclonic-Atonic Seizures. Am. J. Hum. Genet. 2015;96:808–815. doi: 10.1016/j.ajhg.2015.02.016. - DOI - PMC - PubMed
-
- Farhan H., Korkhov V.M., Paulitschke V., Dorostkar M.M., Scholze P., Kudlacek O., Freissmuth M., Sitte H.H. Two Discontinuous Segments in the Carboxyl Terminus Are Required for Membrane Targeting of the Rat Gamma-Aminobutyric Acid Transporter-1 (GAT1) J. Biol. Chem. 2004;279:28553–28563. doi: 10.1074/jbc.M307325200. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

