Recombinant Human Clusterin Seals Damage to the Ocular Surface Barrier in a Mouse Model of Ophthalmic Preservative-Induced Epitheliopathy
- PMID: 36674497
- PMCID: PMC9861099
- DOI: 10.3390/ijms24020981
Recombinant Human Clusterin Seals Damage to the Ocular Surface Barrier in a Mouse Model of Ophthalmic Preservative-Induced Epitheliopathy
Abstract
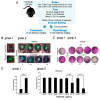
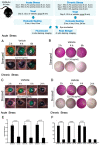
There is a significant unmet need for therapeutics to treat ocular surface barrier damage, also called epitheliopathy, due to dry eye and related diseases. We recently reported that the natural tear glycoprotein CLU (clusterin), a molecular chaperone and matrix metalloproteinase inhibitor, seals and heals epitheliopathy in mice subjected to desiccating stress in a model of aqueous-deficient/evaporative dry eye. Here we investigated CLU sealing using a second model with features of ophthalmic preservative-induced dry eye. The ocular surface was stressed by topical application of the ophthalmic preservative benzalkonium chloride (BAC). Then eyes were treated with CLU and sealing was evaluated immediately by quantification of clinical dye uptake. A commercial recombinant form of human CLU (rhCLU), as well as an rhCLU form produced in our laboratory, designed to be compatible with U.S. Food and Drug Administration guidelines on current Good Manufacturing Practices (cGMP), were as effective as natural plasma-derived human CLU (pCLU) in sealing the damaged ocular surface barrier. In contrast, two other proteins found in tears: TIMP1 and LCN1 (tear lipocalin), exhibited no sealing activity. The efficacy and selectivity of rhCLU for sealing of the damaged ocular surface epithelial barrier suggests that it could be of therapeutic value in treating BAC-induced epitheliopathy and related diseases.
Keywords: clusterin; dry eye; epitheliopathy; matrix metalloproteinase inhibitor; molecular chaperone; ocular surface.
Conflict of interest statement
M.E.F. and S.J. are named as co-inventors on US patent number 9241974 entitled “Clusterin Pharmaceuticals and Treatment Methods Using the Same” granted to the University of Southern California. M.E.F. is a co-founder and chief scientific officer for Proteris Biotech, Inc. M.E.F., S.K.C., M.R.W. and J.T.B. hold equity in Proteris Biotech. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- TFOS Report of the International Dry Eye Workshop (DEWS) Ocul. Surf. 2007;5:65–204. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

