Evaluation of Oxidative Stress and Metabolic Profile in a Preclinical Kidney Transplantation Model According to Different Preservation Modalities
- PMID: 36674540
- PMCID: PMC9861050
- DOI: 10.3390/ijms24021029
Evaluation of Oxidative Stress and Metabolic Profile in a Preclinical Kidney Transplantation Model According to Different Preservation Modalities
Abstract
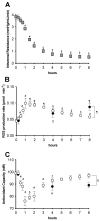
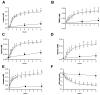
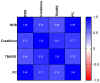
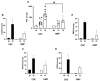
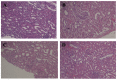
This study addresses a joint nuclear magnetic resonance (NMR) and electron paramagnetic resonance (EPR) spectroscopy approach to provide a platform for dynamic assessment of kidney viability and metabolism. On porcine kidney models, ROS production, oxidative damage kinetics, and metabolic changes occurring both during the period between organ retrieval and implantation and after kidney graft were examined. The 1H-NMR metabolic profile—valine, alanine, acetate, trimetylamine-N-oxide, glutathione, lactate, and the EPR oxidative stress—resulting from ischemia/reperfusion injury after preservation (8 h) by static cold storage (SCS) and ex vivo machine perfusion (HMP) methods were monitored. The functional recovery after transplantation (14 days) was evaluated by serum creatinine (SCr), oxidative stress (ROS), and damage (thiobarbituric-acid-reactive substances and protein carbonyl enzymatic) assessments. At 8 h of preservation storage, a significantly (p < 0.0001) higher ROS production was measured in the SCS vs. HMP group. Significantly higher concentration data (p < 0.05−0.0001) in HMP vs. SCS for all the monitored metabolites were found as well. The HMP group showed a better function recovery. The comparison of the areas under the SCr curves (AUC) returned a significantly smaller (−12.5 %) AUC in the HMP vs. SCS. EPR-ROS concentration (μmol·g−1) from bioptic kidney tissue samples were significantly lower in HMP vs. SCS. The same result was found for the NMR monitored metabolites: lactate: −59.76%, alanine: −43.17%; valine: −58.56%; and TMAO: −77.96%. No changes were observed in either group under light microscopy. In conclusion, a better and more rapid normalization of oxidative stress and functional recovery after transplantation were observed by HMP utilization.
Keywords: 1H-NMR; EPR; ROS; hypothermic machine perfusion; kidney transplant; metabolomic; organ preservation; oxidative damage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Wolfe R.A., Ashby V.B., Milford E.L., Ojo A.O., Ettenger R.E., Agodoa L.Y., Held P.J., Port F.K. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N. Engl. J. Med. 1999;41:1725–1730. doi: 10.1056/NEJM199912023412303. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

