ArtinM Cytotoxicity in B Cells Derived from Non-Hodgkin's Lymphoma Depends on Syk and Src Family Kinases
- PMID: 36674590
- PMCID: PMC9863955
- DOI: 10.3390/ijms24021075
ArtinM Cytotoxicity in B Cells Derived from Non-Hodgkin's Lymphoma Depends on Syk and Src Family Kinases
Abstract
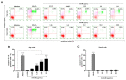
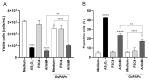
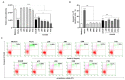
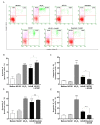
Receptors on the immune cell surface have a variety of glycans that may account for the immunomodulation induced by lectins, which have a carbohydrate recognition domain (CRD) that binds to monosaccharides or oligosaccharides in a specific manner. ArtinM, a D-mannose-binding lectin obtained from Artocarpus heterophyllus, has affinity for the N-glycans core. Immunomodulation by ArtinM toward the Th1 phenotype occurs via its interaction with TLR2/CD14 N-glycans on antigen-presenting cells, as well as recognition of CD3γ N-glycans on murine CD4+ and CD8+ T cells. ArtinM exerts a cytotoxic effect on Jurkat human leukemic T-cell line and human myeloid leukemia cell line (NB4). The current study evaluated the effects of ArtinM on murine and human B cells derived from non-Hodgkin’s lymphoma. We found that murine B cells are recognized by ArtinM via the CRD, and the ArtinM stimulus did not augment the proliferation rate or production of IL-2. However, murine B cell incubation with ArtinM augmented the rate of apoptosis, and this cytotoxic effect of ArtinM was also seen in human B cell-lines sourced from non-Hodgkin’s lymphoma Raji cell line. This cytotoxic effect was inhibited by the phosphatase activity of CD45 on Lck, and the protein kinases of the Src family contribute to cell death triggered by ArtinM.
Keywords: ArtinM; apoptosis; lectin; leukemia; murine and human B cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Mazola Y., Chinea G., Musacchio A. Glycosylation and Bioinformatics: Current Status for Glycosylation Prediction Tools. Biotecnol. Apl. 2011;28:6–12.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

