Gene Therapy in ALS and SMA: Advances, Challenges and Perspectives
- PMID: 36674643
- PMCID: PMC9860634
- DOI: 10.3390/ijms24021130
Gene Therapy in ALS and SMA: Advances, Challenges and Perspectives
Abstract
Gene therapy is defined as the administration of genetic material to modify, manipulate gene expression or alter the properties of living cells for therapeutic purposes. Recent advances and improvements in this field have led to many breakthroughs in the treatment of various diseases. As a result, there has been an increasing interest in the use of these therapies to treat motor neuron diseases (MNDs), for which many potential molecular targets have been discovered. MNDs are neurodegenerative disorders that, in their most severe forms, can lead to respiratory failure and death, for instance, spinal muscular atrophy (SMA) or amyotrophic lateral sclerosis (ALS). Despite the fact that SMA has been known for many years, it is still one of the most common genetic diseases causing infant mortality. The introduction of drugs based on ASOs-nusinersen; small molecules-risdiplam; and replacement therapy (GRT)-Zolgensma has shown a significant improvement in both event-free survival and the quality of life of patients after using these therapies in the available trial results. Although there is still no drug that would effectively alleviate the course of the disease in ALS, the experience gained from SMA gene therapy gives hope for a positive outcome of the efforts to produce an effective and safe drug. The aim of this review is to present current progress and prospects for the use of gene therapy in the treatment of both SMA and ALS.
Keywords: ALS; SMA; gene therapy; motor neuron disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

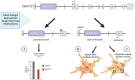
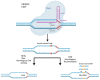
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

