Streptozotocin-Induced Type 1 and 2 Diabetes Mellitus Mouse Models Show Different Functional, Cellular and Molecular Patterns of Diabetic Cardiomyopathy
- PMID: 36674648
- PMCID: PMC9860590
- DOI: 10.3390/ijms24021132
Streptozotocin-Induced Type 1 and 2 Diabetes Mellitus Mouse Models Show Different Functional, Cellular and Molecular Patterns of Diabetic Cardiomyopathy
Abstract
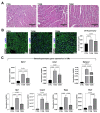
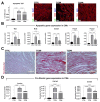
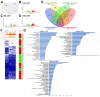
The main cause of morbidity and mortality in diabetes mellitus (DM) is cardiovascular complications. Diabetic cardiomyopathy (DCM) remains incompletely understood. Animal models have been crucial in exploring DCM pathophysiology while identifying potential therapeutic targets. Streptozotocin (STZ) has been widely used to produce experimental models of both type 1 and type 2 DM (T1DM and T2DM). Here, we compared these two models for their effects on cardiac structure, function and transcriptome. Different doses of STZ and diet chows were used to generate T1DM and T2DM in C57BL/6J mice. Normal euglycemic and nonobese sex- and age-matched mice served as controls (CTRL). Immunohistochemistry, RT-PCR and RNA-seq were employed to compare hearts from the three animal groups. STZ-induced T1DM and T2DM affected left ventricular function and myocardial performance differently. T1DM displayed exaggerated apoptotic cardiomyocyte (CM) death and reactive hypertrophy and fibrosis, along with increased cardiac oxidative stress, CM DNA damage and senescence, when compared to T2DM in mice. T1DM and T2DM affected the whole cardiac transcriptome differently. In conclusion, the STZ-induced T1DM and T2DM mouse models showed significant differences in cardiac remodeling, function and the whole transcriptome. These differences could be of key relevance when choosing an animal model to study specific features of DCM.
Keywords: cardiac cell senescence; left ventricular remodeling; myocardial regeneration; streptozotocin; type 1 diabetes mellitus; type 2 diabetes mellitus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

