Data-Driven Approaches Used for Compound Library Design for the Treatment of Parkinson's Disease
- PMID: 36674652
- PMCID: PMC9867512
- DOI: 10.3390/ijms24021134
Data-Driven Approaches Used for Compound Library Design for the Treatment of Parkinson's Disease
Abstract
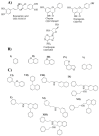
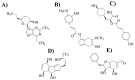
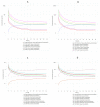
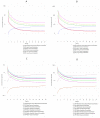
Parkinson's disease (PD) is the second most common neurodegenerative disease in older individuals worldwide. Pharmacological treatment for such a disease consists of drugs such as monoamine oxidase B (MAO-B) inhibitors to increase dopamine concentration in the brain. However, such drugs have adverse reactions that limit their use for extended periods; thus, the design of less toxic and more efficient compounds may be explored. In this context, cheminformatics and computational chemistry have recently contributed to developing new drugs and the search for new therapeutic targets. Therefore, through a data-driven approach, we used cheminformatic tools to find and optimize novel compounds with pharmacological activity against MAO-B for treating PD. First, we retrieved from the literature 3316 original articles published between 2015-2021 that experimentally tested 215 natural compounds against PD. From such compounds, we built a pharmacological network that showed rosmarinic acid, chrysin, naringenin, and cordycepin as the most connected nodes of the network. From such compounds, we performed fingerprinting analysis and developed evolutionary libraries to obtain novel derived structures. We filtered these compounds through a docking test against MAO-B and obtained five derived compounds with higher affinity and lead likeness potential. Then we evaluated its antioxidant and pharmacokinetic potential through a docking analysis (NADPH oxidase and CYP450) and physiologically-based pharmacokinetic (PBPK modeling). Interestingly, only one compound showed dual activity (antioxidant and MAO-B inhibitors) and pharmacokinetic potential to be considered a possible candidate for PD treatment and further experimental analysis.
Keywords: Parkinson’s disease; chemoinformatics; computational drug design; data-driven approach.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

