Brassica napus Roots Use Different Strategies to Respond to Warm Temperatures
- PMID: 36674684
- PMCID: PMC9863162
- DOI: 10.3390/ijms24021143
Brassica napus Roots Use Different Strategies to Respond to Warm Temperatures
Abstract
Elevated growth temperatures are negatively affecting crop productivity by increasing yield losses. The modulation of root traits associated with improved response to rising temperatures is a promising approach to generate new varieties better suited to face the environmental constraints caused by climate change. In this study, we identified several Brassica napus root traits altered in response to warm ambient temperatures. Different combinations of changes in specific root traits result in an extended and deeper root system. This overall root growth expansion facilitates root response by maximizing root-soil surface interaction and increasing roots' ability to explore extended soil areas. We associated these traits with coordinated cellular events, including changes in cell division and elongation rates that drive root growth increases triggered by warm temperatures. Comparative transcriptomic analysis revealed the main genetic determinants of these root system architecture (RSA) changes and uncovered the necessity of a tight regulation of the heat-shock stress response to adjusting root growth to warm temperatures. Our work provides a phenotypic, cellular, and genetic framework of root response to warming temperatures that will help to harness root response mechanisms for crop yield improvement under the future climatic scenario.
Keywords: Brassica napus; climate change; comparative transcriptomic analysis; crop adaptation; heat-shock response; root traits; temperature.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
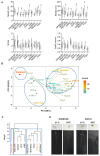


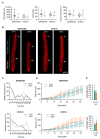

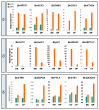

References
-
- Dempewolf H., Eastwood R.J., Guarino L., Khoury C.K., Mller J.V., Toll J. Adapting Agriculture to Climate Change: A Global Initiative to Collect, Conserve, and Use Crop Wild Relatives. Agroecol. Sustain. Food Syst. 2014;38:369–377. doi: 10.1080/21683565.2013.870629. - DOI
-
- Tai A.P.K., Martin M.V., Heald C.L. Threat to future global food security from climate change and ozone air pollution. Nat. Clim. Chang. 2014;4:817–821. doi: 10.1038/nclimate2317. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

