Calcium-Signalling in Human Glaucoma Lamina Cribrosa Myofibroblasts
- PMID: 36674805
- PMCID: PMC9862249
- DOI: 10.3390/ijms24021287
Calcium-Signalling in Human Glaucoma Lamina Cribrosa Myofibroblasts
Abstract
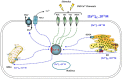
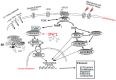
Glaucoma is one of the most common causes of treatable visual impairment in the developed world, affecting approximately 64 million people worldwide, some of whom will be bilaterally blind from irreversible optic nerve damage. The optic nerve head is a key site of damage in glaucoma where there is fibrosis of the connective tissue in the lamina cribrosa (LC) extracellular matrix. As a ubiquitous second messenger, calcium (Ca2+) can interact with various cellular proteins to regulate multiple physiological processes and contribute to a wide range of diseases, including cancer, fibrosis, and glaucoma. Our research has shown evidence of oxidative stress, mitochondrial dysfunction, an elevated expression of Ca2+ entry channels, Ca2+-dependent pumps and exchangers, and an abnormal rise in cytosolic Ca2+ in human glaucomatous LC fibroblast cells. We have evidence that this increase is dependent on Ca2+ entry channels located in the plasma membrane, and its release is from internal stores in the endoplasmic reticulum (ER), as well as from the mitochondria. Here, we summarize some of the molecular Ca2+-dependent mechanisms related to this abnormal Ca2+-signalling in human glaucoma LC cells, with a view toward identifying potential therapeutic targets for ongoing optic neuropathy.
Keywords: calcium homeostasis; fibrosis; glaucoma; lamina cribrosa.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this article.
Figures


References
-
- Hernandez M.R., Igoe F., Neufeld A.H. Cell culture of the human lamina cribrosa. Investig. Ophthalmol. Vis. Sci. 1988;29:78–89. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

