Dapagliflozin Treatment Augments Bioactive Phosphatidylethanolamine Concentrations in Kidney Cortex Membrane Fractions of Hypertensive Diabetic db/db Mice and Alters the Density of Lipid Rafts in Mouse Proximal Tubule Cells
- PMID: 36674924
- PMCID: PMC9865226
- DOI: 10.3390/ijms24021408
Dapagliflozin Treatment Augments Bioactive Phosphatidylethanolamine Concentrations in Kidney Cortex Membrane Fractions of Hypertensive Diabetic db/db Mice and Alters the Density of Lipid Rafts in Mouse Proximal Tubule Cells
Abstract
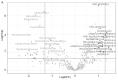
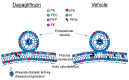
In addition to inhibiting renal glucose reabsorption and allowing for glucose excretion, the sodium/glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin may be efficacious in treating various comorbidities associated with type 2 diabetes mellitus (T2DM). The molecular mechanisms by which dapagliflozin exerts its beneficial effects are largely unknown. We hypothesized dapagliflozin treatment in the diabetic kidney alters plasma membrane lipid composition, suppresses extracellular vesicle (EV) release from kidney cells, and disrupts lipid rafts in proximal tubule cells. In order to test this hypothesis, we treated diabetic db/db mice with dapagliflozin (N = 8) or vehicle (N = 8) and performed mass spectrometry-based lipidomics to investigate changes in the concentrations of membrane lipids in the kidney cortex. In addition, we isolated urinary EVs (uEVs) from urine samples collected during the active phase and the inactive phase of the mice and then probed for changes in membrane proteins enriched in the EVs. Multiple triacylglycerols (TAGs) were enriched in the kidney cortex membrane fractions of vehicle-treated diabetic db/db mice, while the levels of multiple phosphatidylethanolamines were significantly higher in similar mice treated with dapagliflozin. EV concentration and size were lesser in the urine samples collected during the inactive phase of dapagliflozin-treated diabetic mice. In cultured mouse proximal tubule cells treated with dapagliflozin, the lipid raft protein caveolin-1 shifted from less dense fractions to more dense sucrose density gradient fractions. Taken together, these results suggest dapagliflozin may regulate lipid-mediated signal transduction in the diabetic kidney.
Keywords: dapagliflozin; diabetes; kidney; lipid rafts; lipidomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Ishizawa K., Wang Q., Li J., Xu N., Nemoto Y., Morimoto C., Fujii W., Tamura Y., Fujigaki Y., Tsukamoto K., et al. Inhibition of Sodium Glucose Cotransporter 2 Attenuates the Dysregulation of Kelch-Like 3 and NaCl Cotransporter in Obese Diabetic Mice. J. Am. Soc. Nephrol. 2019;30:782–794. doi: 10.1681/ASN.2018070703. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

