Reviewing the Potential Links between Viral Infections and TDP-43 Proteinopathies
- PMID: 36675095
- PMCID: PMC9867397
- DOI: 10.3390/ijms24021581
Reviewing the Potential Links between Viral Infections and TDP-43 Proteinopathies
Abstract
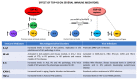
Transactive response DNA binding protein 43 kDa (TDP-43) was discovered in 2001 as a cellular factor capable to inhibit HIV-1 gene expression. Successively, it was brought to new life as the most prevalent RNA-binding protein involved in several neurological disorders, such as amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD). Despite the fact that these two research areas could be considered very distant from each other, in recent years an increasing number of publications pointed out the existence of a potentially important connection. Indeed, the ability of TDP-43 to act as an important regulator of all aspects of RNA metabolism makes this protein also a critical factor during expression of viral RNAs. Here, we summarize all recent observations regarding the involvement of TDP-43 in viral entry, replication and latency in several viruses that include enteroviruses (EVs), Theiler's murine encephalomyelitis virus (TMEV), human immunodeficiency virus (HIV), human endogenous retroviruses (HERVs), hepatitis B virus (HBV), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), West Nile virus (WNV), and herpes simplex virus-2 (HSV). In particular, in this work, we aimed to highlight the presence of similarities with the most commonly studied TDP-43 related neuronal dysfunctions.
Keywords: RNA metabolism; RNA-binding proteins; TDP-43; neuronal dysfunctions; viral entry; viral latency; viral replication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
RNA-binding deficient TDP-43 drives cognitive decline in a mouse model of TDP-43 proteinopathy.Elife. 2023 Oct 11;12:RP85921. doi: 10.7554/eLife.85921. Elife. 2023. PMID: 37819053 Free PMC article.
-
Physiological functions and pathobiology of TDP-43 and FUS/TLS proteins.J Neurochem. 2016 Aug;138 Suppl 1:95-111. doi: 10.1111/jnc.13625. Epub 2016 Jun 15. J Neurochem. 2016. PMID: 27015757 Review.
-
Molecular dissection of TDP-43 proteinopathies.J Mol Neurosci. 2011 Nov;45(3):480-5. doi: 10.1007/s12031-011-9571-x. Epub 2011 Jun 16. J Mol Neurosci. 2011. PMID: 21678031
-
How do the RNA-binding proteins TDP-43 and FUS relate to amyotrophic lateral sclerosis and frontotemporal degeneration, and to each other?Curr Opin Neurol. 2012 Dec;25(6):701-7. doi: 10.1097/WCO.0b013e32835a269b. Curr Opin Neurol. 2012. PMID: 23041957 Review.
-
TDP-43 induces mitochondrial damage and activates the mitochondrial unfolded protein response.PLoS Genet. 2019 May 17;15(5):e1007947. doi: 10.1371/journal.pgen.1007947. eCollection 2019 May. PLoS Genet. 2019. PMID: 31100073 Free PMC article.
Cited by
-
Implications of TDP-43 in non-neuronal systems.Cell Commun Signal. 2023 Nov 23;21(1):338. doi: 10.1186/s12964-023-01336-5. Cell Commun Signal. 2023. PMID: 37996849 Free PMC article. Review.
-
Copper toxicity and deficiency: the vicious cycle at the core of protein aggregation in ALS.Front Mol Neurosci. 2024 Jul 9;17:1408159. doi: 10.3389/fnmol.2024.1408159. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39050823 Free PMC article. Review.
-
Progranulin deficiency in the brain: the interplay between neuronal and non-neuronal cells.Transl Neurodegener. 2025 Apr 16;14(1):18. doi: 10.1186/s40035-025-00475-8. Transl Neurodegener. 2025. PMID: 40234992 Free PMC article. Review.
-
Neurotropic virus infection and neurodegenerative diseases: Potential roles of autophagy pathway.CNS Neurosci Ther. 2024 Jun;30(6):e14548. doi: 10.1111/cns.14548. Epub 2023 Dec 11. CNS Neurosci Ther. 2024. PMID: 38082503 Free PMC article. Review.
-
Molecular Mechanisms Associated with Neurodegeneration of Neurotropic Viral Infection.Mol Neurobiol. 2024 May;61(5):2881-2903. doi: 10.1007/s12035-023-03761-6. Epub 2023 Nov 9. Mol Neurobiol. 2024. PMID: 37946006 Free PMC article. Review.
References
-
- Alami N.H., Smith R.B., Carrasco M.A., Williams L.A., Winborn C.S., Han S.S.W., Kiskinis E., Winborn B., Freibaum B.D., Kanagaraj A., et al. Axonal transport of TDP-43 mRNA granules is impaired by ALS-causing mutations. Neuron. 2014;81:536–543. doi: 10.1016/j.neuron.2013.12.018. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

