Structures and Applications of Nucleic Acid-Based Micelles for Cancer Therapy
- PMID: 36675110
- PMCID: PMC9861421
- DOI: 10.3390/ijms24021592
Structures and Applications of Nucleic Acid-Based Micelles for Cancer Therapy
Abstract
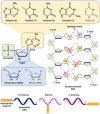
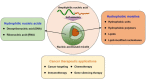
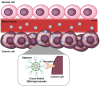
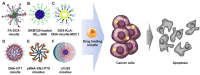
Nucleic acids have become important building blocks in nanotechnology over the last 30 years. DNA and RNA can sequentially build specific nanostructures, resulting in versatile drug delivery systems. Self-assembling amphiphilic nucleic acids, composed of hydrophilic and hydrophobic segments to form micelle structures, have the potential for cancer therapeutics due to their ability to encapsulate hydrophobic agents into their core and position functional groups on the surface. Moreover, DNA or RNA within bio-compatible micelles can function as drugs by themselves. This review introduces and discusses nucleic acid-based spherical micelles from diverse amphiphilic nucleic acids and their applications in cancer therapy.
Keywords: DNAs; RNAs; amphiphiles; chemotherapy; gene silencing; immunotherapy; nanoparticles; targeting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
DNA-π Amphiphiles: A Unique Building Block for the Crafting of DNA-Decorated Unilamellar Nanostructures.Acc Chem Res. 2020 Nov 17;53(11):2668-2679. doi: 10.1021/acs.accounts.0c00492. Epub 2020 Oct 14. Acc Chem Res. 2020. PMID: 33052654
-
Aptamer-based self-assembled nanomicelle enables efficient and targeted drug delivery.J Nanobiotechnology. 2023 Nov 9;21(1):415. doi: 10.1186/s12951-023-02164-y. J Nanobiotechnology. 2023. PMID: 37946192 Free PMC article.
-
Natural and Synthetic Micelles for the Delivery of Small Molecule Drugs, Imaging Agents and Nucleic Acids.Curr Pharm Des. 2022;28(17):1389-1405. doi: 10.2174/1381612828666220506135301. Curr Pharm Des. 2022. PMID: 35524674 Review.
-
Sequence-Defined DNA Amphiphiles for Drug Delivery: Synthesis and Self-Assembly.Methods Mol Biol. 2020;2063:87-100. doi: 10.1007/978-1-0716-0138-9_8. Methods Mol Biol. 2020. PMID: 31667765
-
Sugar-based amphiphilic polymers for biomedical applications: from nanocarriers to therapeutics.Acc Chem Res. 2014 Oct 21;47(10):2867-77. doi: 10.1021/ar4003009. Epub 2014 Aug 20. Acc Chem Res. 2014. PMID: 25141069 Review.
Cited by
-
Nanocarriers for cutting-edge cancer immunotherapies.J Transl Med. 2025 Apr 16;23(1):447. doi: 10.1186/s12967-025-06435-0. J Transl Med. 2025. PMID: 40234928 Free PMC article. Review.
-
A DFT investigation on the potential of beryllium oxide (Be12O12) as a nanocarrier for nucleobases.PLoS One. 2024 Nov 22;19(11):e0313885. doi: 10.1371/journal.pone.0313885. eCollection 2024. PLoS One. 2024. PMID: 39576836 Free PMC article.
-
Decoding interactions between biofilms and DNA nanoparticles.Biofilm. 2025 Feb 6;9:100260. doi: 10.1016/j.bioflm.2025.100260. eCollection 2025 Jun. Biofilm. 2025. PMID: 40026394 Free PMC article.
-
Surfactant-Enabled Nanocarriers in Breast Cancer Therapy: Targeted Delivery and Multidrug Resistance Reversal.Pharmaceutics. 2025 Jun 13;17(6):779. doi: 10.3390/pharmaceutics17060779. Pharmaceutics. 2025. PMID: 40574091 Free PMC article. Review.
References
-
- Neidle S., Sanderson M. Principles of Nucleic Acid Structure. Academic Press; Cambridge, MA, USA: 2021.
-
- Pedersen R., Marchi A.N., Majikes J., Nash J.A., Estrich N.A., Courson D.S., Hall C.K., Craig S.L., LaBean T.H. Properties of DNA. In: Bhushan B., Luo D., Schricker S.R., Sigmund W., Zauscher S., editors. Handbook of Nanomaterials Properties. Springer; Berlin/Heidelberg, Germany: 2014. pp. 1125–1157. - DOI
-
- Watson J.D., Crick F.H. A Structure for Deoxyribose Nucleic Acid. University of Chicago Press; Chicago, IL, USA: 1953.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

