Prospects for Anti-Tumor Mechanism and Potential Clinical Application Based on Glutathione Peroxidase 4 Mediated Ferroptosis
- PMID: 36675129
- PMCID: PMC9864218
- DOI: 10.3390/ijms24021607
Prospects for Anti-Tumor Mechanism and Potential Clinical Application Based on Glutathione Peroxidase 4 Mediated Ferroptosis
Abstract
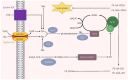
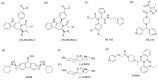
Ferroptosis, characterized by excessive iron accumulation and lipid peroxidation, is a novel form of iron-dependent cell death, which is morphologically, genetically, and biochemically distinct from other known cell death types, such as apoptosis, necrosis, and autophagy. Emerging evidence shows that glutathione peroxidase 4 (GPX4), a critical core regulator of ferroptosis, plays an essential role in protecting cells from ferroptosis by removing the product of iron-dependent lipid peroxidation. The fast-growing studies on ferroptosis in cancer have boosted a perspective on its use in cancer therapeutics. In addition, significant progress has been made in researching and developing tumor therapeutic drugs targeting GPX4 based on ferroptosis, especially in acquired drug resistance. Selenium modulates GPX4-mediated ferroptosis, and its existing form, selenocysteine (Sec), is the active center of GPX4. This review explored the structure and function of GPX4, with the overarching goal of revealing its mechanism and potential application in tumor therapy through regulating ferroptosis. A deeper understanding of the mechanism and application of GPX4-mediated ferroptosis in cancer therapy will provide new strategies for the research and development of antitumor drugs.
Keywords: anti-tumor; cancer; ferroptosis; glutathione peroxidase 4; selenocysteine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Targeting GPX4 in human cancer: Implications of ferroptosis induction for tackling cancer resilience.Cancer Lett. 2023 Apr 10;559:216119. doi: 10.1016/j.canlet.2023.216119. Epub 2023 Mar 8. Cancer Lett. 2023. PMID: 36893895 Review.
-
Significance of glutathione peroxidase 4 and intracellular iron level in ovarian cancer cells-"utilization" of ferroptosis mechanism.Inflamm Res. 2021 Dec;70(10-12):1177-1189. doi: 10.1007/s00011-021-01495-6. Epub 2021 Sep 19. Inflamm Res. 2021. PMID: 34537856
-
Mechanisms and regulations of ferroptosis.Front Immunol. 2023 Oct 6;14:1269451. doi: 10.3389/fimmu.2023.1269451. eCollection 2023. Front Immunol. 2023. PMID: 37868994 Free PMC article. Review.
-
Oxytosis/Ferroptosis in Neurodegeneration: the Underlying Role of Master Regulator Glutathione Peroxidase 4 (GPX4).Mol Neurobiol. 2024 Mar;61(3):1507-1526. doi: 10.1007/s12035-023-03646-8. Epub 2023 Sep 19. Mol Neurobiol. 2024. PMID: 37725216 Review.
-
Lipid Peroxidation-Dependent Cell Death Regulated by GPx4 and Ferroptosis.Curr Top Microbiol Immunol. 2017;403:143-170. doi: 10.1007/82_2016_508. Curr Top Microbiol Immunol. 2017. PMID: 28204974 Review.
Cited by
-
Esketamine alleviated cardiomyocyte ferroptosis induced by oxygen-glucose deprivation/reoxygenation (OGD/R) via cyclic GMP-AMP synthase interactor.Cytotechnology. 2025 Apr;77(2):57. doi: 10.1007/s10616-025-00723-9. Epub 2025 Feb 8. Cytotechnology. 2025. PMID: 39931675
-
The ferroptosis-related gene GGTLC2 is identified as a novel biomarker for gastric cancer within the GGT family, with associations to immune infiltration and liver metastasis.Funct Integr Genomics. 2025 May 21;25(1):106. doi: 10.1007/s10142-025-01614-0. Funct Integr Genomics. 2025. PMID: 40397220
-
Glutathione-Dependent Pathways in Cancer Cells.Int J Mol Sci. 2024 Aug 1;25(15):8423. doi: 10.3390/ijms25158423. Int J Mol Sci. 2024. PMID: 39125992 Free PMC article. Review.
-
Inhibition of FSP1: A new strategy for the treatment of tumors (Review).Oncol Rep. 2024 Aug;52(2):105. doi: 10.3892/or.2024.8764. Epub 2024 Jun 28. Oncol Rep. 2024. PMID: 38940330 Free PMC article. Review.
-
Resistance to FOXM1 inhibitors in breast cancer is accompanied by impeding ferroptosis and apoptotic cell death.Breast Cancer Res Treat. 2024 Nov;208(2):307-320. doi: 10.1007/s10549-024-07420-9. Epub 2024 Jul 9. Breast Cancer Res Treat. 2024. PMID: 38980505 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

