The Old and the New: Cardiovascular and Respiratory Alterations Induced by Acute JWH-018 Administration Compared to Δ9-THC-A Preclinical Study in Mice
- PMID: 36675144
- PMCID: PMC9865969
- DOI: 10.3390/ijms24021631
The Old and the New: Cardiovascular and Respiratory Alterations Induced by Acute JWH-018 Administration Compared to Δ9-THC-A Preclinical Study in Mice
Abstract
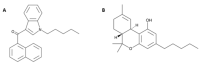
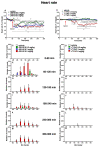
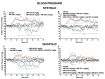
Several new psychoactive substances (NPS) are responsible for intoxication involving the cardiovascular and respiratory systems. Among NPS, synthetic cannabinoids (SCs) provoked side effects in humans characterized by tachycardia, arrhythmias, hypertension, breathing difficulty, apnoea, myocardial infarction, and cardiac arrest. Therefore, the present study investigated the cardio-respiratory (MouseOx Plus; EMKA electrocardiogram (ECG) and plethysmography TUNNEL systems) and vascular (BP-2000 systems) effects induced by 1-naphthalenyl (1-pentyl-1H-indol-3-yl)-methanone (JWH-018; 0.3-3-6 mg/kg) and Δ9-tetrahydrocannabinol (Δ9-THC; 0.3-3-6 mg/kg), administered in awake CD-1 male mice. The results showed that higher doses of JWH-018 (3-6 mg/kg) induced deep and long-lasting bradycardia, alternated with bradyarrhythmia, spaced out by sudden episodes of tachyarrhythmias (6 mg/kg), and characterized by ECG electrical parameters changes, sustained bradypnea, and systolic and transient diastolic hypertension. Otherwise, Δ9-THC provoked delayed bradycardia (minor intensity tachyarrhythmias episodes) and bradypnea, also causing a transient and mild hypertensive effect at the tested dose range. These effects were prevented by both treatment with selective CB1 (AM 251, 6 mg/kg) and CB2 (AM 630, 6 mg/kg) receptor antagonists and with the mixture of the antagonists AM 251 and AM 630, even if in a different manner. Cardio-respiratory and vascular symptoms could be induced by peripheral and central CB1 and CB2 receptors stimulation, which could lead to both sympathetic and parasympathetic systems activation. These findings may represent a starting point for necessary future studies aimed at exploring the proper antidotal therapy to be used in SCs-intoxicated patient management.
Keywords: blood pressure; cardiovascular; plethysmography; synthetic cannabinoid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











Similar articles
-
JWH-018 impairs sensorimotor functions in mice.Neuroscience. 2015 Aug 6;300:174-88. doi: 10.1016/j.neuroscience.2015.05.021. Epub 2015 May 15. Neuroscience. 2015. PMID: 25987201
-
Cannabinoids in disguise: Δ9-tetrahydrocannabinol-like effects of tetramethylcyclopropyl ketone indoles.Neuropharmacology. 2013 Dec;75:145-54. doi: 10.1016/j.neuropharm.2013.07.022. Epub 2013 Aug 2. Neuropharmacology. 2013. PMID: 23916483 Free PMC article.
-
Different receptor mechanisms underlying phytocannabinoid- versus synthetic cannabinoid-induced tetrad effects: Opposite roles of CB1 /CB2 versus GPR55 receptors.Br J Pharmacol. 2020 Apr;177(8):1865-1880. doi: 10.1111/bph.14958. Epub 2020 Feb 11. Br J Pharmacol. 2020. PMID: 31877572 Free PMC article.
-
The synthetic CB1 cannabinoid receptor selective agonists: Putative medical uses and their legalization.Prog Neuropsychopharmacol Biol Psychiatry. 2021 Aug 30;110:110301. doi: 10.1016/j.pnpbp.2021.110301. Epub 2021 Mar 17. Prog Neuropsychopharmacol Biol Psychiatry. 2021. PMID: 33741446 Review.
-
Distinct pharmacology and metabolism of K2 synthetic cannabinoids compared to Δ(9)-THC: mechanism underlying greater toxicity?Life Sci. 2014 Feb 27;97(1):45-54. doi: 10.1016/j.lfs.2013.09.017. Epub 2013 Sep 29. Life Sci. 2014. PMID: 24084047 Free PMC article. Review.
Cited by
-
Synthetic cannabinoid receptor agonists exacerbate fentanyl-elicited respiratory depression and confer resistance to naloxone rescue in mice.Drug Alcohol Depend. 2025 Jul 1;272:112672. doi: 10.1016/j.drugalcdep.2025.112672. Epub 2025 Apr 15. Drug Alcohol Depend. 2025. PMID: 40319790
-
Tackling new psychoactive substances through metabolomics: UHPLC-HRMS study on natural and synthetic opioids in male and female murine models.Sci Rep. 2024 Apr 24;14(1):9432. doi: 10.1038/s41598-024-60045-2. Sci Rep. 2024. PMID: 38658766 Free PMC article.
-
Hexahydrocannabinol: pharmacokinetics, systemic toxicity, and acute behavioral effects in Wistar rats.Int J Neuropsychopharmacol. 2025 Aug 1;28(8):pyaf041. doi: 10.1093/ijnp/pyaf041. Int J Neuropsychopharmacol. 2025. PMID: 40643195 Free PMC article.
-
The Dark Side of "Smart Drugs": Cognitive Enhancement vs. Clinical Concerns.Toxics. 2025 Mar 26;13(4):247. doi: 10.3390/toxics13040247. Toxics. 2025. PMID: 40278563 Free PMC article. Review.
-
Δ9-Tetrahydrocannabinol Effects on Respiration and Heart Rate Across Route of Administration in Female and Male Mice.Cardiovasc Toxicol. 2023 Dec;23(11-12):349-363. doi: 10.1007/s12012-023-09810-9. Epub 2023 Sep 20. Cardiovasc Toxicol. 2023. PMID: 37728714 Free PMC article.
References
-
- United Nations Office on Drugs and Crime (UNODC) The Challenge of New Psychoactive Substances: A Report from the Global SMART Programme: Vienna. 2013. [(accessed on 6 June 2022)]. Available online: https://www.unodc.org/documents/scientific/NPS_2013_SMART.pdf.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

