ONC201 Suppresses Neuroblastoma Growth by Interrupting Mitochondrial Function and Reactivating Nuclear ATRX Expression While Decreasing MYCN
- PMID: 36675163
- PMCID: PMC9867473
- DOI: 10.3390/ijms24021649
ONC201 Suppresses Neuroblastoma Growth by Interrupting Mitochondrial Function and Reactivating Nuclear ATRX Expression While Decreasing MYCN
Abstract
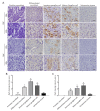
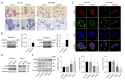
Neuroblastoma (NB) is characterized by several malignant phenotypes that are difficult to treat effectively without combination therapy. The therapeutic implication of mitochondrial ClpXP protease ClpP and ClpX has been verified in several malignancies, but is unknown in NB. Firstly, we observed a significant increase in ClpP and ClpX expression in immature and mature ganglion cells as compared to more malignant neuroblasts and less malignant Schwannian-stroma-dominant cell types in human neuroblastoma tissues. We used ONC201 targeting ClpXP to treat NB cells, and found a significant suppression of mitochondrial protease, i.e., ClpP and ClpX, expression and downregulation of mitochondrial respiratory chain subunits SDHB and NDUFS1. The latter was associated with a state of energy depletion, increased reactive oxygen species, and decreased mitochondrial membrane potential, consequently promoting apoptosis and suppressing cell growth of NB. Treatment of NB cells with ONC201 as well as the genetic attenuation of ClpP and ClpX through specific short interfering RNA (siRNA) resulted in the significant upregulation of the tumor suppressor alpha thalassemia/mental retardation X-linked (ATRX) and promotion of neurite outgrowth, implicating mitochondrial ClpXP proteases in MYCN-amplified NB cell differentiation. Furthermore, ONC201 treatment significantly decreased MYCN protein expression and suppressed tumor formation with the reactivation of ATRX expression in MYCN-amplified NB-cell-derived xenograft tumors. Taken together, ONC201 could be the potential agent to provide diversified therapeutic application in NB, particularly in NB with MYCN amplification.
Keywords: ATRX; ClpPX protease; MYCN; ONC201; apoptosis; mitochondria; neuroblastoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




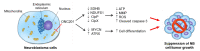
References
-
- Chuang J.H., Chou M.H., Tai M.H., Lin T.K., Liou C.W., Chen T., Hsu W.M., Wang P.W. 2-Deoxyglucose treatment complements the cisplatin- or BH3-only mimetic-induced suppression of neuroblastoma cell growth. Int. J. Biochem. Cell Biol. 2013;45:944–951. doi: 10.1016/j.biocel.2013.01.019. - DOI - PubMed
-
- Huang C.C., Wang S.Y., Lin L.L., Wang P.W., Chen T.Y., Hsu W.M., Lin T.K., Liou C.W., Chuang J.H. Glycolytic inhibitor 2-deoxyglucose simultaneously targets cancer and endothelial cells to suppress neuroblastoma growth in mice. Dis. Model Mech. 2015;8:1247–1254. doi: 10.1242/dmm.021667. - DOI - PMC - PubMed
-
- Schibler J., Tomanek-Chalkley A.M., Reedy J.L., Zhan F., Spitz D.R., Schultz M.K., Goel A. Mitochondrial-Targeted Decyl-Triphenylphosphonium Enhances 2-Deoxy-D-Glucose Mediated Oxidative Stress and Clonogenic Killing of Multiple Myeloma Cells. PLoS ONE. 2016;11:e0167323. doi: 10.1371/journal.pone.0167323. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

