Synthesis of Fluorescent, Dumbbell-Shaped Polyurethane Homo- and Heterodendrimers and Their Photophysical Properties
- PMID: 36675178
- PMCID: PMC9866862
- DOI: 10.3390/ijms24021662
Synthesis of Fluorescent, Dumbbell-Shaped Polyurethane Homo- and Heterodendrimers and Their Photophysical Properties
Abstract
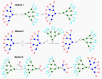
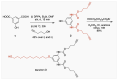
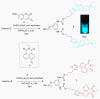
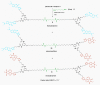
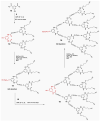
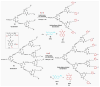
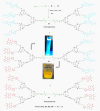
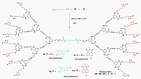
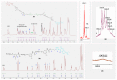
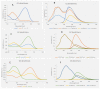
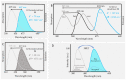
Fluorescent dendrimers have wide applications in biomedical and materials science. Here, we report the synthesis of fluorescent polyurethane homodendrimers and Janus dendrimers, which often pose challenges due to the inherent reactivity of isocyanates. Polyurethane dendrons (G1-G3) were synthesized via a convergent method using a one-pot multicomponent Curtius reaction as a crucial step to establish urethane linkages. The alkyne periphery of the G1-G3 dendrons was modified by a copper catalyzed azide-alkyne click reaction (CuAAC) to form fluorescent dendrons. In the reaction of the surfaces functionalized two different dendrons with a difunctional core, a mixture of three dendrimers consisting of two homodendrimers and a Janus dendrimer were obtained. The Janus dendrimer accounted for a higher proportion in the products' distribution, being as high as 93% for G3. The photophysical properties of Janus dendrimers showed the fluorescence resonance energy transfer (FRET) from one to the other fluorophore of the dendrimer. The FRET observation accompanied by a large Stokes shift make these dendrimers potential candidates for the detection and tracking of interactions between the biomolecules, as well as potential candidates for fluorescence imaging.
Keywords: Janus dendrimers; absorption; click reaction; emission; fluorescence; heterodendrimers; homodendrimers; late-stage modification; polyurethane dendrimers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
A Model for Late-Stage Modification of Polyurethane Dendrimers Using Thiol-Ene Click Chemistry.ACS Omega. 2021 Apr 29;6(18):12375-12381. doi: 10.1021/acsomega.1c01609. eCollection 2021 May 11. ACS Omega. 2021. PMID: 34056389 Free PMC article.
-
Low-generation fluorescent polyurethane dendrimers via late-stage modification using azide-alkyne click chemistry.RSC Adv. 2022 Oct 3;12(43):28043-28051. doi: 10.1039/d2ra04937f. eCollection 2022 Sep 28. RSC Adv. 2022. PMID: 36320285 Free PMC article.
-
Synthesis and characterization of poly(amido amine) dendrimer containing fluorene as a core chromophore.J Nanosci Nanotechnol. 2008 Sep;8(9):4635-9. doi: 10.1166/jnn.2008.ic59. J Nanosci Nanotechnol. 2008. PMID: 19049074
-
Click dendrimers and triazole-related aspects: catalysts, mechanism, synthesis, and functions. A bridge between dendritic architectures and nanomaterials.Acc Chem Res. 2012 Apr 17;45(4):630-40. doi: 10.1021/ar200235m. Epub 2011 Dec 8. Acc Chem Res. 2012. PMID: 22148925 Review.
-
Synthesis and applications of biomedical and pharmaceutical polymers via click chemistry methodologies.Bioconjug Chem. 2009 Nov;20(11):2001-16. doi: 10.1021/bc900087a. Bioconjug Chem. 2009. PMID: 19606898 Review.
Cited by
-
Sensing and Microbiological Activity of a New Blue Fluorescence Polyamidoamine Dendrimer Modified with 1,8-Naphthalimide Units.Molecules. 2024 Apr 25;29(9):1960. doi: 10.3390/molecules29091960. Molecules. 2024. PMID: 38731451 Free PMC article.
References
-
- Caminade A.M., Laurent R., Delavaux-Nicot B., Majoral J.P. “Janus” Dendrimers: Syntheses and Properties. New J. Chem. 2012;36:217–226. doi: 10.1039/C1NJ20458K. - DOI
-
- Duan Y., Zhao X., Sun M., Hao H. Research Advances in the Synthesis, Application, Assembly, and Calculation of Janus Materials. Ind. Eng. Chem. Res. 2021;60:1071–1095. doi: 10.1021/acs.iecr.0c04304. - DOI
-
- Liu X., Gitsov I. Thermosensitive Amphiphilic Janus Dendrimers with Embedded Metal Binding Sites. Synthesis and Self-Assembly. Macromolecules. 2018;51:5085–5100. doi: 10.1021/acs.macromol.8b00700. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

