Nebulized Menthol Impairs Mucociliary Clearance via TRPM8 and MUC5AC/MUC5B in Primary Airway Epithelial Cells
- PMID: 36675209
- PMCID: PMC9865048
- DOI: 10.3390/ijms24021694
Nebulized Menthol Impairs Mucociliary Clearance via TRPM8 and MUC5AC/MUC5B in Primary Airway Epithelial Cells
Abstract
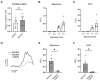
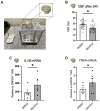
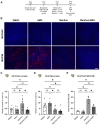
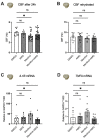
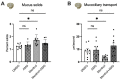
Flavorings enhance the palatability of e-cigarettes (e-cigs), with menthol remaining a popular choice among e-cig users. Menthol flavor remains one of the only flavors approved by the United States FDA for use in commercially available, pod-based e-cigs. However, the safety of inhaled menthol at the high concentrations used in e-cigs remains unclear. Here, we tested the effects of menthol on parameters of mucociliary clearance (MCC) in air-liquid interface (ALI) cultures of primary airway epithelial cells. ALI cultures treated with basolateral menthol (1 mM) showed a significant decrease in ciliary beat frequency (CBF) and airway surface liquid (ASL) volumes after 24 h. Menthol nebulized onto the surface of ALI cultures similarly reduced CBF and increased mucus concentrations, resulting in decreased rates of mucociliary transport. Nebulized menthol further increased the expression of mucin 5AC (MUC5AC) and mRNA expression of the inflammatory cytokines IL1B and TNFA. Menthol activated TRPM8, and the effects of menthol on MCC and inflammation could be blocked by a specific TRPM8 antagonist. These data provide further evidence that menthol at the concentrations used in e-cigs could cause harm to the airways.
Keywords: MUC5AC; MUC5B; TRPM8; airway epithelium; menthol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Clapp P.W., Lavrich K.S., van Heusden C.A., Lazarowski E.R., Carson J.L., Jaspers I. Cinnamaldehyde in flavored e-cigarette liquids temporarily suppresses bronchial epithelial cell ciliary motility by dysregulation of mitochondrial function. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2019;316:L470–L486. doi: 10.1152/ajplung.00304.2018. - DOI - PMC - PubMed
-
- Park H.R., O’Sullivan M., Vallarino J., Shumyatcher M., Himes B.E., Park J.A., Christiani D.C., Allen J., Lu Q. Transcriptomic response of primary human airway epithelial cells to flavoring chemicals in electronic cigarettes. Sci. Rep. 2019;9:1400. doi: 10.1038/s41598-018-37913-9. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

