Salicylanilides and Their Anticancer Properties
- PMID: 36675241
- PMCID: PMC9861143
- DOI: 10.3390/ijms24021728
Salicylanilides and Their Anticancer Properties
Abstract
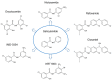
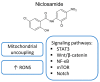
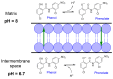
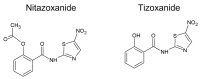
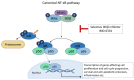
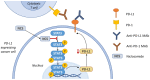
Salicylanilides are pharmacologically active compounds with a wide spectrum of biological effects. Halogenated salicylanilides, which have been used for decades in human and veterinary medicine as anthelmintics, have recently emerged as candidates for drug repurposing in oncology. The most prominent example of salicylanilide anthelmintic, that is intensively studied for its potential anticancer properties, is niclosamide. Nevertheless, recent studies have discovered extensive anticancer potential in a number of other salicylanilides. This potential of their anticancer action is mediated most likely by diverse mechanisms of action such as uncoupling of oxidative phosphorylation, inhibition of protein tyrosine kinase epidermal growth factor receptor, modulation of different signaling pathways as Wnt/β-catenin, mTORC1, STAT3, NF-κB and Notch signaling pathways or induction of B-Raf V600E inhibition. Here we provide a comprehensive overview of the current knowledge about the proposed mechanisms of action of anticancer activity of salicylanilides based on preclinical in vitro and in vivo studies, or structural requirements for such an activity.
Keywords: STAT3; TK EGFR; anticancer properties; drug repurposing; mitochondrial uncoupling; niclosamide; salicylanilides.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
The Antifungal Potential of Niclosamide and Structurally Related Salicylanilides.Int J Mol Sci. 2024 May 29;25(11):5977. doi: 10.3390/ijms25115977. Int J Mol Sci. 2024. PMID: 38892165 Free PMC article. Review.
-
The pharmacology of halogenated salicylanilides and their anthelmintic use in animals.J S Afr Vet Assoc. 1999 Jun;70(2):61-70. doi: 10.4102/jsava.v70i2.756. J S Afr Vet Assoc. 1999. PMID: 10855824 Review.
-
[The preparation and antihymenolepic activity of a bromine-containing salicylanilide the compound MST-16)].Med Parazitol (Mosk). 2014 Jul-Sep;(3):31-2. Med Parazitol (Mosk). 2014. PMID: 25286549 Russian. No abstract available.
-
[A novel bromine-containing salicylanilide (the compound MST-18). Preparation and antihymenolepic activity].Med Parazitol (Mosk). 2014 Jul-Sep;(3):30-1. Med Parazitol (Mosk). 2014. PMID: 25286548 Russian. No abstract available.
-
Mechanistic Understanding Enables the Rational Design of Salicylanilide Combination Therapies for Gram-Negative Infections.mBio. 2020 Sep 15;11(5):e02068-20. doi: 10.1128/mBio.02068-20. mBio. 2020. PMID: 32934086 Free PMC article.
Cited by
-
Repositioning anthelmintics for the treatment of inflammatory-based pathological conditions.Inflammopharmacology. 2025 Feb;33(2):551-571. doi: 10.1007/s10787-024-01605-w. Epub 2024 Nov 26. Inflammopharmacology. 2025. PMID: 39589670 Review.
-
Impact of Oncogenic Changes in p53 and KRAS on Macropinocytosis and Ferroptosis in Colon Cancer Cells and Anticancer Efficacy of Niclosamide with Differential Effects on These Two Processes.Cells. 2024 May 30;13(11):951. doi: 10.3390/cells13110951. Cells. 2024. PMID: 38891084 Free PMC article.
-
Superior Anticancer and Antifungal Activities of New Sulfanyl-Substituted Niclosamide Derivatives.Biomedicines. 2024 Jul 21;12(7):1621. doi: 10.3390/biomedicines12071621. Biomedicines. 2024. PMID: 39062194 Free PMC article.
-
The Antifungal Potential of Niclosamide and Structurally Related Salicylanilides.Int J Mol Sci. 2024 May 29;25(11):5977. doi: 10.3390/ijms25115977. Int J Mol Sci. 2024. PMID: 38892165 Free PMC article. Review.
-
Optimizing Niclosamide for Cancer Therapy: Improving Bioavailability via Structural Modification and Nanotechnology.Cancers (Basel). 2024 Oct 21;16(20):3548. doi: 10.3390/cancers16203548. Cancers (Basel). 2024. PMID: 39456642 Free PMC article. Review.
References
-
- Alasadi A., Chen M., Swapna G.V.T., Tao H.L., Guo J.J., Collantes J., Fadhil N., Montelione G.T., Jin S.K. Effect of mitochondrial uncouplers niclosamide ethanolamine (NEN) and oxyclozanide on hepatic metastasis of colon cancer. Cell Death Dis. 2018;9:215. doi: 10.1038/s41419-017-0092-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

