Handheld Ultrasound or Conventional Ultrasound Devices in Patients Undergoing HCT: A Validation Study
- PMID: 36675449
- PMCID: PMC9867323
- DOI: 10.3390/jcm12020520
Handheld Ultrasound or Conventional Ultrasound Devices in Patients Undergoing HCT: A Validation Study
Abstract
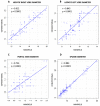
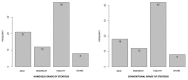
Abdominal ultrasound exams play a major role in the diagnosis of sinusoidal obstruction syndrome/veno-occlusive disease (SOS/VOD). The development of portable hand-held ultrasound devices (HHUS) has been shown to facilitate the diagnosis of many diseases, but little data on the value of HHUS in the diagnosis of SOS/VOD are available. We performed a study aimed at validating portable ultrasound (US) devices in the setting of hematopoietic stem cell transplant (HCT). Sixteen evaluable patients undergoing allogeneic HCT were studied using conventional US and HHUS during the first 3 weeks after transplant. The results obtained demonstrate that there is a close correlation between conventional and handheld ultrasound examination in the measurement of the right hepatic lobe (r = 0.912, p < 0.0001), the left hepatic lobe (r = 0.843, p < 0.0001), the portal vein (PV) (r = 0.724, p < 0.0001), and the spleen (r = 0.983, p < 0.0001) based on Pearson’s correlation. The same data, analyzed through Lin’s concordance correlation coefficient, evidenced a substantial level of agreement in the comparison of the spleen and right hepatic lobe, while a lower grade of agreement in the measurement of the portal vein and left hepatic lobe. Moreover, there was good agreement between results obtained by the two types of ultrasound devices in assessing ascites (p < 0.0001), gallbladder thickening (p < 0.0001), and the direction of PV flow (p < 0.0001). HHUS device allows the study of HokUs-10 parameters with an excellent agreement with conventional US, and may contribute to SOS/VOD diagnosis.
Keywords: handheld ultrasound device; hematopoietic stem cell transplantation; liver veno-occlusive disease.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Shulman H.M., McDonald G.B., Matthews D., Doney K.C., Kopecky K.J., Gauvreau J.M., Thomas E.D. An analysis of hepatic veno-occlusive disease and centrilobular hepatic degeneration following bone marrow transplantation. Gastroenterology. 1980;79:1178–1191. doi: 10.1016/0016-5085(80)90911-7. - DOI - PubMed
-
- Carreras E., Bertz H., Arcese W., Vernant J.-P., Tomás J.-F., Hagglund H., Bandini G., Esperou H., Russell J., de la Rubia J., et al. Incidence and Outcome of Hepatic Veno-Occlusive Disease After Blood or Marrow Transplantation: A Prospective Cohort Study of the European Group for Blood and Marrow Transplantation. Blood. 1998;92:3599–3604. doi: 10.1182/BLOOD.V92.10.3599. - DOI - PubMed
-
- Kernan N.A., Grupp S., Smith A.R., Arai S., Triplett B., Antin J.H., Lehmann L., Shore T., Ho V.T., Bunin N., et al. Final results from a defibrotide treatment-IND study for patients with hepatic veno-occlusive disease/sinusoidal obstruction syndrome. Br. J. Haematol. 2018;181:816–827. doi: 10.1111/bjh.15267. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

