Severe Intrahepatic Cholestasis of Pregnancy-Potential Mechanism by Which Fetuses Are Protected from the Hazardous Effect of Bile Acids
- PMID: 36675545
- PMCID: PMC9860676
- DOI: 10.3390/jcm12020616
Severe Intrahepatic Cholestasis of Pregnancy-Potential Mechanism by Which Fetuses Are Protected from the Hazardous Effect of Bile Acids
Abstract
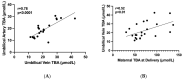
Intrahepatic cholestasis of pregnancy (ICP) is characterized by elevated total bile acids (TBA). Although elevated maternal TBA is a major risk factors for fetal morbidity and mortality, it is unclear why some fetuses are more prone to the hazardous effect of bile acids (BA) over the others. It is unclear whether fetuses are protected by placental BA uptake, or it is the fetal BA metabolism that reduces fetal BA as compared to maternal levels. Therefore, we aimed to compared TBA levels in the umbilical vein and artery to maternal TBA in women with ICP. The study included 18 women who had TBA > 40 μmol/L and their 23 fetuses. We found that the TBA level in umbilical vein was significantly lower compared to maternal TBA level. The TBA levels in umbilical vein and umbilical artery were similar. No fetus had a serious neonatal complication. Importantly, since TBA level remains low even though maternal TBA level is high the fetuses are protected from the hazardous effects of maternal BA. Our findings suggest that there is no effective metabolism of BA in the fetus and the main decrease in TBA in the fetus is related to placental BA uptake.
Keywords: intrahepatic cholestasis of pregnancy; placenta; total bile acid; ursodeoxycholic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

