Sterile Cerebrospinal Fluid Culture at Cryptococcal Meningitis Diagnosis Is Associated with High Mortality
- PMID: 36675867
- PMCID: PMC9866844
- DOI: 10.3390/jof9010046
Sterile Cerebrospinal Fluid Culture at Cryptococcal Meningitis Diagnosis Is Associated with High Mortality
Abstract
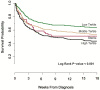
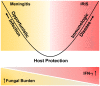
Cryptococcus is the leading cause of AIDS-related meningitis in sub-Saharan Africa. The clinical implications of a sterile cerebrospinal fluid (CSF) culture among individuals diagnosed with cryptococcal meningitis using CSF cryptococcal antigen (CrAg) are unclear. We prospectively enrolled 765 HIV-positive Ugandans with first-episode cryptococcal meningitis from November 2010 to May 2017. All persons were treated with amphotericin-based induction therapy. We grouped participants by tertile of baseline CSF quantitative Cryptococcus culture burden and compared clinical characteristics, CSF immune profiles, and 18-week mortality. We found 55 (7%) CSF CrAg-positive participants with sterile CSF cultures. Compared to the non-sterile groups, participants with sterile CSF cultures had higher CD4 counts, lower CSF opening pressures, and were more frequently receiving ART. By 18 weeks, 47% [26/55] died in the sterile culture group versus 35% [83/235] in the low culture tertile, 46% [107/234] in the middle tertile, and 56% [135/241] in the high tertile (p < 0.001). The sterile group had higher levels of CSF interferon-gamma (IFN-γ), IFN-α, interleukin (IL)-6, IL-17, G-CSF, GM-CSF, and chemokine CXCL2 compared with non-sterile groups. Despite persons with sterile CSF cultures having higher CD4 counts, lower CSF opening pressures, and CSF cytokine profiles associated with better Cryptococcus control (e.g., IFN-γ predominant), mortality was similar to those with higher fungal burdens. This unexpected finding challenges the traditional paradigm that increasing CSF fungal burdens are associated with increased mortality but is consistent with a damage-response framework model.
Keywords: AIDS; Cryptococcus; HIV; cryptococcal meningitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ellis J., Bangdiwala A.S., Cresswell F.V., Rhein J., Nuwagira E., Ssebambulidde K., Tugume L., Rajasingham R., Bridge S.C., Muzoora C., et al. The Changing Epidemiology of HIV-Associated Adult Meningitis, Uganda 2015–2017. Open Forum Infect. Dis. 2019;6:ofz419. doi: 10.1093/ofid/ofz419. - DOI - PMC - PubMed
-
- Rajasingham R., Govender N.P., Jordan A., Loyse A., Shroufi A., Denning D.W., Meya D.B., Chiller T.M., Boulware D.R. The global burden of HIV-associated cryptococcal infection in adults in 2020: A modelling analysis. Lancet Infect. Dis. 2022;22:1748–1755. doi: 10.1016/S1473-3099(22)00499-6. - DOI - PMC - PubMed
-
- Boulware D.R., Rolfes M.A., Rajasingham R., von Hohenberg M., Qin Z., Taseera K., Schutz C., Kwizera R., Butler E.K., Meintjes G., et al. Multisite validation of cryptococcal antigen lateral flow assay and quantification by laser thermal contrast. Emerg. Infect. Dis. 2014;20:45–53. doi: 10.3201/eid2001.130906. - DOI - PMC - PubMed
-
- Jarvis J.N., Bicanic T., Loyse A., Namarika D., Jackson A., Nussbaum J.C., Longley N., Muzoora C., Phulusa J., Taseera K., et al. Determinants of mortality in a combined cohort of 501 patients with HIV-associated Cryptococcal meningitis: Implications for improving outcomes. Clin. Infect. Dis. 2014;58:736–745. doi: 10.1093/cid/cit794. - DOI - PMC - PubMed
Grants and funding
- U01 AI089244/AI/NIAID NIH HHS/United States
- K23 AI138851/AI/NIAID NIH HHS/United States
- UL1TR002494/TR/NCATS NIH HHS/United States
- K01 TW010268/TW/FIC NIH HHS/United States
- KL2 TR002492/TR/NCATS NIH HHS/United States
- KL2TR002492/TR/NCATS NIH HHS/United States
- T32 AI055433/AI/NIAID NIH HHS/United States
- K01TW010268/TW/FIC NIH HHS/United States
- K43 TW010718/TW/FIC NIH HHS/United States
- R01 NS086312/NS/NINDS NIH HHS/United States
- UL1 TR002494/TR/NCATS NIH HHS/United States
- R01NS086312/NS/NINDS NIH HHS/United States
- K43TW010718/TW/FIC NIH HHS/United States
- K23 NS122601/NS/NINDS NIH HHS/United States
- D43TW009345/TW/FIC NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

